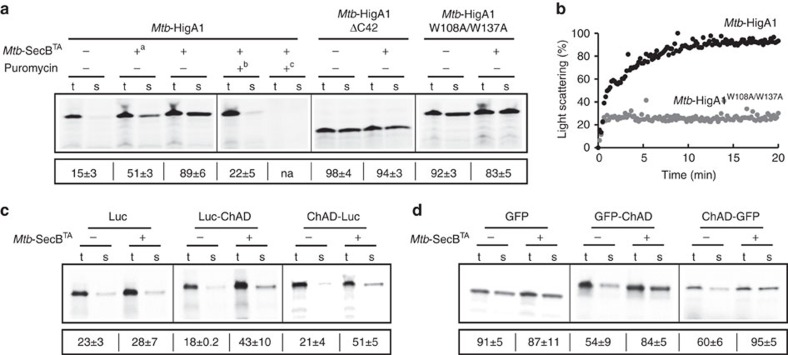
Figure 2. The chaperone facilitates the folding of Mtb-HigA1 and other unrelated proteins only in the presence of ChAD.
(a) Folding of newly translated Mtb-HigA1 relies on Mtb-SecBTA when the ChAD extension of Mtb-HigA1 is present. Mtb-HigA1, Mtb-HigA1ΔC42 and Mtb-HigA1W108A/W137A were independently expressed in a cell-free translation system with or without Mtb-SecBTA. Translation products were labelled with [35S]methionine. Mtb-SecBTA concentrations were 8 μM otherwise stated (+a indicates 4 μM) and reactions were performed for 1 h at 37 °C. When indicated, 0.4 mM of puromycin was added, either from the start of the translation reaction (+c) or 30 min after (+b), to release ribosome-bound nascent chains for 1 h, and the chaperone was then added for 1 h at 37 °C. After translation, the total (t) and soluble (s) fractions were separated on SDS–PAGE and quantified by phosphorimager. The numbers below the electrophoretic pattern represent the mean solubility values (%), calculated by the ratio of the amount of translation products in the soluble (s) and total (t) fractions obtained from three different translation experiments. The s.d. is indicated. (b) In vitro aggregation kinetics of urea-denatured Mtb-HigA1 and Mtb-HigA1W108A/W137A (4 μM) followed at 25 °C by monitoring light scattering at 355 nm. (c) Effect of Mtb-SecBTA on the solubility of nascent luciferase (Luc) and chimeric luciferase containing C-terminal ChAD (Luc-ChAD) or N-terminal ChAD-luciferase (ChAD-Luc) and (d) on the solubility of nascent GFP and chimeric GFP-C-terminal ChAD (GFP-ChAD) and N-terminal ChAD-GFP (ChAD-GFP), as performed in a, with or without Mtb-SecBTA (8 μM). Full phosphorimager images for a,c and d are shown in Supplementary Fig. 8.