Abstract
Aim:
The aim of our study was to assess the limitation of clinical examination in determining the morphology of thyroid gland in patients with hyperthyroidism and its implications.
Methods:
A retrospective analysis of consecutive patients with hyperthyroidism seen in a tertiary endocrine clinic were analyzed. Sub-analysis was performed on patients with proven Graves’ disease.
Results:
Of the 133 patients included in this study with hyperthyroidism, 60 (45%) patients had significant nodularity on ultrasound (US). However, only 67% of these were identified on clinical examination. In patients with confirmed Graves’ disease (n = 73), the discordance between US and clinical examination was very similar (18 of 30 patients, 60%).
Conclusion:
US should form an essential part of the evaluation of hyperthyroidism as the morphology of thyroid gland could be variable and nodules in these glands would also need to be appropriately investigated. This would also significantly influence decision-making and appropriate immediate and follow-up management plan.
Key words: Graves’ disease, hyperthyroidism, thyroid examination, thyroid nodules
INTRODUCTION
Hyperthyroidism is a common clinical presentation encountered in endocrine clinics. The approach to diagnosis and management of hyperthyroidism is quite variable in clinical practice with varying dependence on clinical assessment, thyroid autoantibodies, and ultrasound (US) examination. It is quite common that reliance for etiological diagnosis of hyperthyroidism is largely based on clinical assessment and followed by antibody testing. The initial assessment of these patients is quite variable and is generally guided by individual clinician's expertise and experience.[1] The morphology of thyroid gland is generally determined by clinical examination and recommending US of the neck is not a routine clinical practice. The incidence of cancers in palpable thyroid nodule is approximately 3–10%; the risk in incidentally found nonpalpable nodules is quite variable. There is a general clinical belief that hyperthyroid gland is protected against thyroid cancers. However, there has been increasing evidence to the contrary, making it even more pertinent to determine the morphology of the gland accurately.[2] The aim of our study was to assess the reliability of clinical examination of the thyroid gland by endocrine specialists in real-life clinical practice in comparison to US features and reemphasize the need for US as an objective documentation of the thyroid anatomy in patients with hyperthyroidism.
METHODS
We conducted an observational analysis of clinical records of 200 consecutive patients referred to our endocrine clinic for assessment of biochemically confirmed hyperthyroidism. The patients were assessed in the clinic by consultants or specialist registrars in the endocrine clinics. All patients had a clinical history suggestive of hyperthyroidism and biochemical evidence of hyperthyroid state (low or suppressed thyroid stimulating hormone and high free T4 and/or T3). As per the local practice, US examinations were only requested if clinically warranted and at the discretion of the attending endocrinologist. Autoantibodies (thyroid peroxidase [TPO] and/or thyroid receptor antibody) were tested in patients with suspected Graves’ disease. The US of the neck was performed by dedicated consultants or specialist registrars in radiology. The clinical letters were reviewed to document the morphology of the gland on clinical examination.
For the purpose of the analysis, the US findings were classified as:
Normal (in size and shape)
Solitary nodule
Two large nodules (>1 cm each)
Multinodular goiter (MNG) with enlargement of thyroid gland
Dominant nodule in an MNG
Others (which included diffuse enlargement with no nodularity, asymmetrical enlargement, or present of micronodularity with thyroiditis).
RESULTS
Of the 200 patients that were reviewed for the study, 133 were included for the study based on the availability of US report. The mean age was 47 years (21–87). The results of the clinical and US examination are summarized in Table 1.
Table 1.
Clinical findings versus ultrasound findings in patients with hyperthyroidism
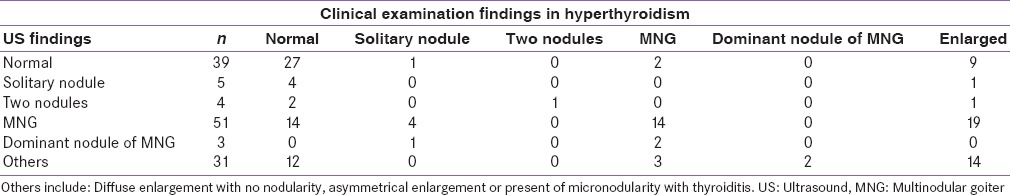
Thirty-nine patients with hyperthyroidism had a normal thyroid gland on US. Twenty-seven of the 39 patients were identified as normal on clinical examination also. Fifty-one patients were found a have an MNG on the US. 14/51 were reported as normal on clinical examination. A further 14 patients were correctly identified as having an MNG and 19 more to have an enlarged gland. Five patients had solitary nodule on the US. 4/5 were classified as normal on clinical examination. Therefore, of the sixty patients who had significant nodularity on US, twenty were classified as normal and hence may not have been referred for US in routine clinical practice.
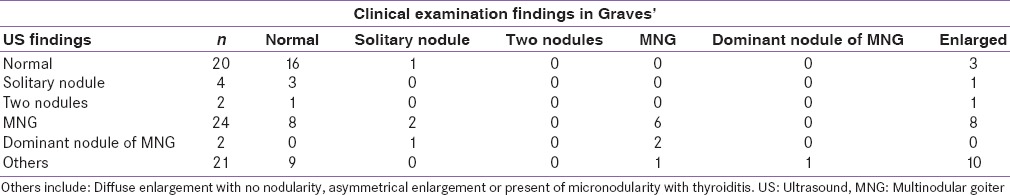
We did a sub-analysis on patients who had antibody confirmed diagnosis of Graves’ disease (n = 73) [Table 2]. It was again noted that 12/30 patients could be misdiagnosed as being normal on clinical examination when the US shows evidence of significant nodularity.
Table 2.
Clinical findings versus ultrasound findings in patients with proven Graves’ disease
DISCUSSION
Our study clearly demonstrates the limitation of clinical examination of the neck in identifying the morphology of the thyroid gland in patients with hyperthyroidism. About 33% of the patients with hyperthyroidism were classified as normal on clinical examination when the US identified nodularity that may warrant further investigation. Our study also highlights a few important clinical issues in the management of patients with hyperthyroidism and Graves’ disease.
(i) Our analysis again demonstrates the limitation of clinical thyroid palpation. The concordance rate of clinical examination versus US was only 56/133 in hyperthyroidism in general and 32/73 in Graves’. There is significant variation in normal clinical practice of requesting US for these patients. A recent survey conducted on practicing endocrinologist in Europe showed an increasing trend toward using US for morphological diagnosis of the thyroid gland.[3] The sensitivity of identifying the nodules reduces with the decreasing size of the nodules; however, clinical examination is not sensitive in identifying all larger nodules as well.[4] (ii) Patients with nodular thyroid gland and hyperthyroidism warrant further investigation with radionuclide uptake scans to document if the nodule is “hot” or “cold.” The estimated prevalence of thyroid cancer in hot nodules is variable but can be as high as 3.1%.[5] The majority of these tend to be papillary thyroid cancers. The prevalence of follicular thyroid cancer is understandably higher in hot nodules in compared to cold nodules (36.4% vs. 10%).[5] The practice recommendations on thyroid nodules recommend using the US imaging to guide the need for biopsy of these nodules.[6,7] The prevalence of thyroid cancers may be low in “hot” nodules; however, the “cold” nodules may still need to be investigated in line with practice with euthyroid nodules in the thyroid gland. Furthermore, once remission is achieved with anti-thyroid treatment, the nodules would warrant continued follow-up if appropriate in line with the general management of thyroid nodules. The usual clinical notion that patients with hyperthyroidism and a nodular gland would need a thyroid uptake scan and if the nodule is hot, risk of malignancy is low, is questionable. These nodules may also need to be investigated if appropriate based on clinical and ultrasonography appearance.[6] (iii) In practices where thyroid receptor antibody or TPO antibodies are not performed as a routine, the role of US is even more vital to prove the etiology of hyperthyroidism. This is crucial to decide the duration of treatment of anti-thyroid drugs and for assessing the need for earlier definitive treatment.
In patients with diagnosed Graves’ disease, the morphology of the thyroid gland could be very variable. There are quite a few important points to take into consideration (i) Coexistence of nodules in Graves’ disease is quite common, some present at diagnosis and some developing since the onset of the disease.[8,9] (ii) The risk of thyroid cancer developing in a thyroid gland with Graves’ disease, based on epidemiological studies, is considered to be significantly higher compared to background population and the risk is much higher in the first 3 years after diagnosis.[10] (iii) Thyroid cancers developing in a gland with Graves’ disease could be more aggressive [11] and multifocal malignancy in comparison to toxic adenoma or MNG.[2,12] Although many of these conclusions are derived from thyroidectomy studies (and hence the carcinoma might have only been incidentally identified and of questionable clinical validity), it is important to acknowledge this fact when taking an individual patient into consideration, especially if risk factors are present. (iv) The presence of nodules in a thyroid gland with Graves’ increases the risk of thyroid cancer in comparison to patients with diffuse goiter, thereby again negating the common belief that “hot” nodules are low risk for thyroid cancers.[2] (v) The chance of developing thyroid cancer is higher in a patient with Graves’ disease with a cold nodule in comparison to a toxic adenoma or a hot nodule in an MNG.[13] The above findings therefore increasingly validate the need for performing the routine US as standard practice in patients with Graves’ disease.
There are limitations to our study: This study is a retrospective analysis of standard clinical practice hence adjustment for techniques used in the clinical examination, or inter-rater variability of US cannot be possible. The thyroid function test results were not published as part of the study as this would not have made a difference to the results of the conclusion of the study. The age of the patients and the characteristics of the nodule may influence the need (or lack of) to investigate some of the patients, and this could not be accounted for in our study. There could be variation in epidemiology depending on the iodine levels and iodine intake of a specific population.
CONCLUSION
Our study reemphasizes the need to use a triangulated approach to patients with hyperthyroidism, using clinical examination, antibody testing, and US to derive an etiological diagnosis of hyperthyroidism. Patients with hyperthyroidism and nodules in the thyroid gland would warrant appropriate further investigations as the risk of thyroid cancer in these nodules are not as low as it had been thought of.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement:
We would like to thank Dr Quratul-Ain Altaf, Consultant Endocrinologist, Heart of England NHS Trust, UK, for her efforts in the preliminary data collection for this study.
REFERENCES
- 1.Wartofsky L, Glinoer D, Solomon B, Nagataki S, Lagasse R, Nagayama Y, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1:129–35. doi: 10.1089/thy.1991.1.129. [DOI] [PubMed] [Google Scholar]
- 2.Pazaitou-Panayiotou K, Michalakis K, Paschke R. Thyroid cancer in patients with hyperthyroidism. Horm Metab Res. 2012;44:255–62. doi: 10.1055/s-0031-1299741. [DOI] [PubMed] [Google Scholar]
- 3.Bartalena L, Burch HB, Burman KD, Kahaly GJ. A 2013 European survey of clinical practice patterns in the management of Graves’ disease. Clin Endocrinol (Oxf) 2016;84:115–20. doi: 10.1111/cen.12688. [DOI] [PubMed] [Google Scholar]
- 4.Wiest PW, Hartshorne MF, Inskip PD, Crooks LA, Vela BS, Telepak RJ, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17:487–96. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 5.Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: Review of the literature. Thyroid Res. 2013;6:7. doi: 10.1186/1756-6614-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 7.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81(Suppl 1):1–122. doi: 10.1111/cen.12515. [DOI] [PubMed] [Google Scholar]
- 8.Cantalamessa L, Baldini M, Orsatti A, Meroni L, Amodei V, Castagnone D. Thyroid nodules in Graves disease and the risk of thyroid carcinoma. Arch Intern Med. 1999;159:1705–8. doi: 10.1001/archinte.159.15.1705. [DOI] [PubMed] [Google Scholar]
- 9.Pichaipillai L, Mishra B. The diagnosis of Graves’ disease: Diagnostic and management dilemmas. Endocr Abstr. 2014;34:P401. [Google Scholar]
- 10.Chen YK, Lin CL, Chang YJ, Cheng FT, Peng CL, Sung FC, et al. Cancer risk in patients with Graves’ disease: A nationwide cohort study. Thyroid. 2013;23:879–84. doi: 10.1089/thy.2012.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegriti G, Belfiore A, Giuffrida D, Lupo L, Vigneri R. Outcome of differentiated thyroid cancer in Graves’ patients. J Clin Endocrinol Metab. 1998;83:2805–9. doi: 10.1210/jcem.83.8.4997. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore A, Garofalo MR, Giuffrida D, Runello F, Filetti S, Fiumara A, et al. Increased aggressiveness of thyroid cancer in patients with Graves’ disease. J Clin Endocrinol Metab. 1990;70:830–5. doi: 10.1210/jcem-70-4-830. [DOI] [PubMed] [Google Scholar]
- 13.Cappelli C, Braga M, De Martino E, Castellano M, Gandossi E, Agosti B, et al. Outcome of patients surgically treated for various forms of hyperthyroidism with differentiated thyroid cancer: Experience at an endocrine center in Italy. Surg Today. 2006;36:125–30. doi: 10.1007/s00595-005-3115-3. [DOI] [PubMed] [Google Scholar]


