Summary
The aim of this study is to develop a new technology for making traditional Lithuanian non-alcoholic beverage kvass from fermented cereals by extending the spectrum of raw materials (extruded rye) and applying new biotechnological resources (xylanolytic enzymes and lactic acid bacteria (LAB)) to improve its functional properties. Arabinoxylans in extruded rye were very efficiently hydrolysed into oligosaccharides by xylanolytic complex Ceremix Plus MG. Using Ceremix Plus MG and LAB fermentation, the yield of arabinoxylooligosaccharides and xylooligosaccharides in beverage was increased to 300 and 1100 mg/L, respectively. Beverages fermented by LAB had lower pH values and ethanol volume fraction compared to the yeast-fermented beverage. The acceptability of the beverage fermented by Lactobacillus sakei was higher than of Pediococcus pentosaceus- or yeast- -fermented beverages and similar to the acceptability of commercial kvass made from malt extract. The results showed that extruded rye, xylanolytic enzymes and LAB can be used for production of novel and safe high-value non-alcoholic beverages.
Key words: non-alcoholic beverage, extruded rye, lactic acid bacteria (LAB), xylanase, oligosaccharides
Introduction
Fermented foods and beverages are produced worldwide using various manufacturing techniques, raw materials and microorganisms (1). The problem is that production methods for most of traditional fermented foods are not optimised or industrialised, and are rather small- -scale, localised processes. It is beneficial to revisit such traditional products and to conduct biotechnological investigations to explore and enhance their functional properties, and thus promote their commercial exploitation outside of their local market.
Kvass is a cereal-based beverage traditionally produced from fermented rye and barley malt, rye flour, and stale rye bread in Lithuania and other eastern European countries. It is a non-alcoholic beverage, so the ethanol content should be negligible, and it is considered to be spoiled if alcohol accumulates to higher levels. As opposed to sourdough bread, kvass undergoes no heat processing after fermentation and thus contains high cell counts of viable yeast and lactic acid bacteria (LAB). The microflora of kvass fermentation is normally composed of Saccharomyces cerevisiae and LAB, but the composition of species is expected to be quite variable due to differences in fermentation techniques and fermentation media. To date, microbial associations in kvass fermentation have been poorly investigated (2). Efforts to use pure starter cultures of LAB to produce acceptable kvass have not been documented.
LAB play a key role in food fermentations where they not only contribute to the development of the desired sensory properties in the final product but also to their microbiological safety (3). The antimicrobial effect of LAB is mainly related to the production of organic acids; however, some strains are able to synthesise antimicrobial substances – bacteriocins (3). During recent years the interest in bacteriocin-producing LAB has increased because of their potential to produce natural antimicrobial agents to enhance the safety of food products. The application of such LAB for non-alcoholic cereal-based beverage production provides new opportunities for development of functional fermented products.
Cereals are important source of hemicelluloses, which include arabinoxylans, β-glucans and arabinogalactans, also called dietary fibre (4). Among commonly grown cereals, rye is considered to be a healthy cereal with its high content of dietary fibre and associated components. The growing interest in foods and beverages with increased dietary fibre content is observed worldwide. Such foods and beverages are often called prebiotic foods, because of their ability to stimulate the growth of beneficial colonic microflora (5, 6). Research on prebiotics and other novel health-promoting food components has been intensive for over a decade. Arabinoxylan-derived arabinoxylooligosaccharides (AXOS), which may have various chemical structures, depending on the xylan source and the degradation method used, stand increasingly in the spotlight as potential prebiotics (7, 8). During the past decade, the studies of the possibilities to produce the AXOS by using biocatalytic conversion have received more attention. The enzymatic hydrolysis allows control of the end-products and to obtain AXOS having the desired degree of polymerization and substitutions (9). Therefore, there is an interest in new enzymatic technologies for partial and selective degradation of arabinoxylan (AX).
Most recently, extrusion technology has been used in the development of novel, value-added foods (10, 11). Extrusion cooking is preferable to other food-processing techniques because it is a continuous process with high productivity and significant nutrient retention, owing to the high temperature and short time required. During extrusion cooking, the raw materials undergo many chemical and structural transformations, such as starch gelatinization, protein denaturation, complex formation between amylose and lipids (12). No significant changes were found in dietary fibre content at mild or moderate extrusion conditions, but some fibre components could be solubilised (13). In addition, the extrusion denatures undesirable enzymes, inactivates some antinutritional factors, destructs naturally present toxins, and sterilises the final product (14). The application of new raw materials in the extrusion process opens perspectives both for the increase of the assortment of extruded products and the development of novel components for food industry that exhibit specific technological properties.
The aim of this study is to develop a new technology for making traditional Lithuanian non-alcoholic beverage kvass from fermented cereals by extending the spectrum of raw materials such as extruded rye and applying new biotechnological resources such as xylanolytic enzymes and antimicrobially active LAB to improve the functional properties.
Materials and Methods
Raw materials and enzymes
Rye wholemeal extruded by a single-screw extruder was supplied by the miller Ustukiu Malunas (Pasvalys, Lithuania). Extrusion conditions were: feed rate 110 kg/h, temperature 45/65/110 °C (individual zones), screw speed 350–410 rpm. The extruded rye flour was passed through a 0.5-mm sieve.
Four commercial xylanolytic enzyme preparations were used for degradation of AX to AXOS and XOS: Ceremix Plus MG derived from Humicola insolens and Bacillus licheniformis, Pentopan Mono BG from Thermomyces lanuginosus (both from Novozymes A/S, Bagsvćrd, Denmark), Depol 680P from Bacillus spp. and Aspergillus spp. and Depol 692L from Trichoderma reesei and Aspergillus spp. (both from Biocatalysts Ltd., Parc Nantgarw, Wales, UK). Additionally, Bakezyme P500, an α-amylase preparation from Aspergillus oryzae (DSM, Heerlen, The Netherlands) and AMG 300L, an amyloglucosidase preparation from Aspergillus niger (Novozymes A/S) were used for rye mash liquefaction.
Activity profiles of the enzyme preparations used in the study were determined before the experiment. β-Xylanase activity was measured using the method of Bailey et al. (15) with 1% birchwood xylan (Sigma-Aldrich, Steinheim, Germany) as a substrate. β-Glucanase activity was measured with medium viscosity barley β-glucan 1% (Megazyme International Ireland, Bray, Ireland) as a substrate according to Zurbriggen et al. (16). The α-arabinosidase, β-xylosidase and β-glucosidase activities were measured according to Poutanen et al. (17) and Poutanen and Puls (18) using 2 mM p-nitrophenyl-α--l-arabinofuranoside, 5 mM p-nitrophenyl-β-d-xylopyranoside and 1 mM p-nitrophenyl-β-d-glucopyranoside (Sigma--Aldrich) substrates, respectively. High β-xylanase activity with only low side activities of β-glucanase and β-glucosidase was found in Pentopan Mono BG. Ceremix Plus MG, Depol 680P and Depol 692L preparations contained high β-glucanase activity with side activities of β-xylanase, β-xylosidase (except Depol 692L), α-arabinosidase and β-glucosidase.
Lactic acid bacteria and yeast
Lactobacillus sakei KTU05-6 (optimal temperature 30 °C) and Pediococcus pentosaceus KTU05-10 (optimal temperature 37 °C) from culture collection of the Department of Food Science and Technology (Kaunas University of Technology, Kaunas, Lithuania) were used for beverage fermentation. Tested LAB were isolated from spontaneous rye sourdough and selected due to their high antimicrobial activity (19). LAB were cultured in MRS broth (CM0359; Oxoid Ltd, Hampshire, UK) at appropriate temperatures for 48 h prior to use. For parallel studies dry baker’s yeast Saccharomyces cerevisiae (Fermentis, Marcq-en-Baroeul, France) was used.
Arabinoxylan enzymatic hydrolysis
For hydrolysis of AX, 2.5 mg of each rye sample were suspended in 1 mL of 0.1 M sodium acetate buffer (pH=5) and incubated with four different enzyme preparations separately (600 xylanase units (XU) per g of cereals) at 55 °C for 5 h. Enzymatic reaction was stopped by heating the reaction mixture at 100 °C for 10 min. The degree of AX degradation was determined by high-performance anion--exchange chromatography (HPAEC).
Preparation of beverages
Modified technological scheme for traditional kvass production was used to prepare a non-alcoholic beverage from extruded rye under laboratory conditions. The beverage mash was prepared from extruded wholemeal rye and tap water (55 °C) at a ratio of 1:50 and treated by enzymatic hydrolysis. Simultaneous enzymatic liquefaction and saccharification step was performed in 2 h at 55 °C by adding selected amounts of α-amylase (48.6 amylase units (AU) per g of cereals) and amyloglucosidase (20 glucosidase units (GU) per g); additionally β-xylanolytic enzyme preparation Ceremix Plus MG (12.5 XU/g) was added for the degradation of non-starch polysaccharides. The activities of enzymes were selected according to the producer’s recommendations and the results of initial tests. After liquefaction, the mash was boiled at 100 °C for 10 min to inactivate the enzymes, and cooled down to a temperature suitable for LAB (30 and 37 °C) or yeast (30 °C). The mash was inoculated with 10% of starter culture suspension (108 colony-forming units (CFU) per g, moisture content 65%) or dry yeast (0.6 g per L of mash). Fermentation was carried out for 48–60 h and samples for oligo- and monosaccharide analysis were collected every 12 h and filtered with Eppendorf filtration system (Eppendorf, Hamburg, Germany). Sweetness of fermented mash was adjusted by adding sugar, and caramelised malt was used to obtain brown colour of the drink. The beverage was then cooled, filtered and samples were taken for quality evaluation. The rest of the beverage was dispensed into 250-mL amber glass bottles. The bottles were closed with screw caps and stored at 0–6 °C for 30 days for microbial analysis. Kvass produced from commercial rye malt extract and fermented by yeast under the same conditions served as the control. All fermentations were performed in duplicates.
Chromatographic analysis of saccharides
HPAEC analysis of oligosaccharides extracted from extruded rye was performed on HPLC system, equipped with Waters 717 Plus autosampler, two Waters 515 HPLC pumps (Waters, Milford, MA, USA), analytical column CarboPac PA-100 (250 mm×4 mm) and guard column CarboPac PA-100 Guard (50 mm×4 mm; Dionex, Sunnyvale, CA, USA). Decade pulsed amperometric detector (PAD) (Waters) with a gold electrode (Antec Leyden, Zoeterwoude, The Netherlands) was used in pulse mode at 30 °C and Millenium 32 software (Waters) for instrument control and data handling.
The HPAEC-PAD system for determination of monosaccharides was equipped with Waters 2707 autosampler, three Waters 515 HPLC pumps, analytical column CarboPac PA-1 (250 mm×4 mm) and guard column CarboPac PA-1 Guard (50 mm×4 mm; Dionex), Waters 2465 pulse amperometric detector (PAD) and Empower 2 software (Waters). The mobile phases were filtered through 0.45- -µm GH Polypro membrane filters (Pall Corporation, Port Washington, NY, USA) and degassed using helium.
The eluents for gradient analysis of the oligosaccharides were A: 1 M sodium acetate in 100 mM sodium hydroxide (Sigma-Aldrich) and B: 100 mM sodium hydroxide. The first isocratic phase was 15 min in 100% B, followed by a linear gradient from 100% B to 88% B and 12% A in 20 min. The second isocratic phase was 5 min in 88% B and 12% A. The second linear gradient was back to 100% B in 5 min. The last isocratic phase was 5 min in 100% B, in which the column was stabilised before the next injection. The total analysis time was 50 min and the flow rate was 1 mL/min.
The eluents for gradient analysis of the monosaccharides were A: 200 mM sodium hydroxide and B: water. The first isocratic phase was 13 min in 18% A and 82% B, followed by a linear gradient to 100% A in 9 min. The second isocratic phase was 15 min in 100% A. The second linear gradient was back to 18% A and 82% B in 8 min. The last isocratic phase was 10 min in 18% A and 82% B. The total analysis time was 55 min and the flow rate was 1 mL/min.
External standards were used in both oligo- and monosaccharide analysis for accurate identification and quantification of the formed sugars. For oligosaccharide analysis, the mixtures of XOS (from X2 to X6) and maltooligosaccharides (from M3 to M7) (Megazyme International Ireland) were used. AXOS (A3X, A2X, A2XX, A2,3X, A2,3XX) were prepared at the University of Helsinki, Finland. The preparation, purification and identification of these AXOS are described by Rantanen et al. (20) and Pastell et al. (21). The amount of isolated AXOS was analysed in those papers and these values were used as standard to quantify unknown AXOS mixtures. Additionally, maltose, cellobiose, 1,3-1,4-β-glucotriose, two glucotetroses (different in structure) and galactotetrose were used (Megazyme International Ireland). The peak heights (mV) were used in the quantification of carbohydrates. For the monosaccharides, an external standard mixture containing arabinose, glucose, fructose, xylose (Merck KGaA, Darmstadt, Germany) and sucrose (Sigma-Aldrich) was used. The external standard mixture was injected after every four samples in both methods to monitor the retention times and detection responses.
Beverage quality evaluation
Total titratable acidity (TTA) was analysed by titration with 0.1 M NaOH solution, using phenolphtalein as indicator, and expressed in degrees. One degree (1°) of acidity corresponds to 1 mL of 1 M NaOH required to neutralise the organic acids present in 100 mL of sample. The pH value was measured and recorded using a pH electrode (PP-15; Sartorius, Goettingen, Germany). Volatile acid content was determined by steam distillation and after titration with 0.1 M NaOH it was expressed in g of acetic acid per L. Concentration of lactic acid (g/L) was calculated from the differences between TTA and volatile acidity.
Contents of ethanol (% by volume) and extractable materials (% by mass) were determined after distillation by densitometric method using pycnometer at 20 °C. By-products of fermentation potentially considered as flavour compounds or as impurities (methanol, higher alcohols and esters) were analysed using a Hewlett-Packard 5890 gas chromatograph (Hewlett-Packard, Wilmington, DE, USA) equipped with a split-splitless injector and a flame ionisation detector (FID). The injection temperature was 200 °C and helium served as a carrier gas with a flow rate of 1.2 mL/min. Compounds were separated on a column Zebron ZB-WAX (30 m×0.25 mm×0.25 μm, 100% polyethylene glycol; Phenomenex, Torrance, CA, USA). The temperature program used was: initial temperature of 40 °C for 5 min, rising to 100 °C at a rate of 4 °C/min, and a 2 min hold. The detection temperature was set at 250 °C. Qualitative analysis was done by comparison of retention times of the chromatogram of the kvass samples to that of the standard solution of 6 alcohols and esters (methyl acetate, ethyl acetate, methanol, propanol, isobutanol, isoamyl alcohol; Sigma-Aldrich). A standard solution was prepared by dissolving the analysed compounds in 96% ethanol. Quantitative analysis was conducted using generated calibration curves.
Microbial analysis of beverages
The effect of LAB on the microbiological characteristics of beverages was determined by counting their number and the total number of microorganisms after 1, 10, 20 and 30 days of storage. LAB counts were determined on MRS agar (Liofilchem, Roseto degli Abruzzi, Teramo, Italy) using plate count techniques. Plates were incubated for (72±4) h under anaerobic conditions (using atmosphere generation system AnaeroGen; Oxoid, Basingstoke, UK). The experiment was carried out in triplicate. Microbiological contamination of fermented products was evaluated by calculating the total number of microorganisms and was expressed in CFU/mL using plate count agar (Liofilchem) according to ISO 4833-2:2013 method (22).
Sensory evaluations of the beverages
Sensory evaluations were conducted by 11 panellists, consisting of KTU staff and students, using a 7-point rating scale for the colour, odour and flavour attributes. Value of 1 corresponded to the lowest intensity and of 7 to the highest intensity of the attribute. Overall acceptability was evaluated using a 9-point hedonic scale where 9 is ‘like extremely’ and 1 ‘dislike extremely’. Coded samples were served and water was provided for rinsing between the sensory evaluations of the samples.
Statistical analysis
All analytical determinations were performed at least in triplicates. Results were expressed as mean value±standard deviation (S.D.). Statistical data analysis was conducted using a Microsoft Excel spreadsheet, and the statistical program for Windows (SPSS, v. 16.0; IBM Corporation, Armonk, NY, USA) was used for comparison of the mean values by one-way analysis of variance. The results were significant at p<0.05.
Results and Discussion
Effect of enzyme preparations on rye arabinoxylan hydrolysis
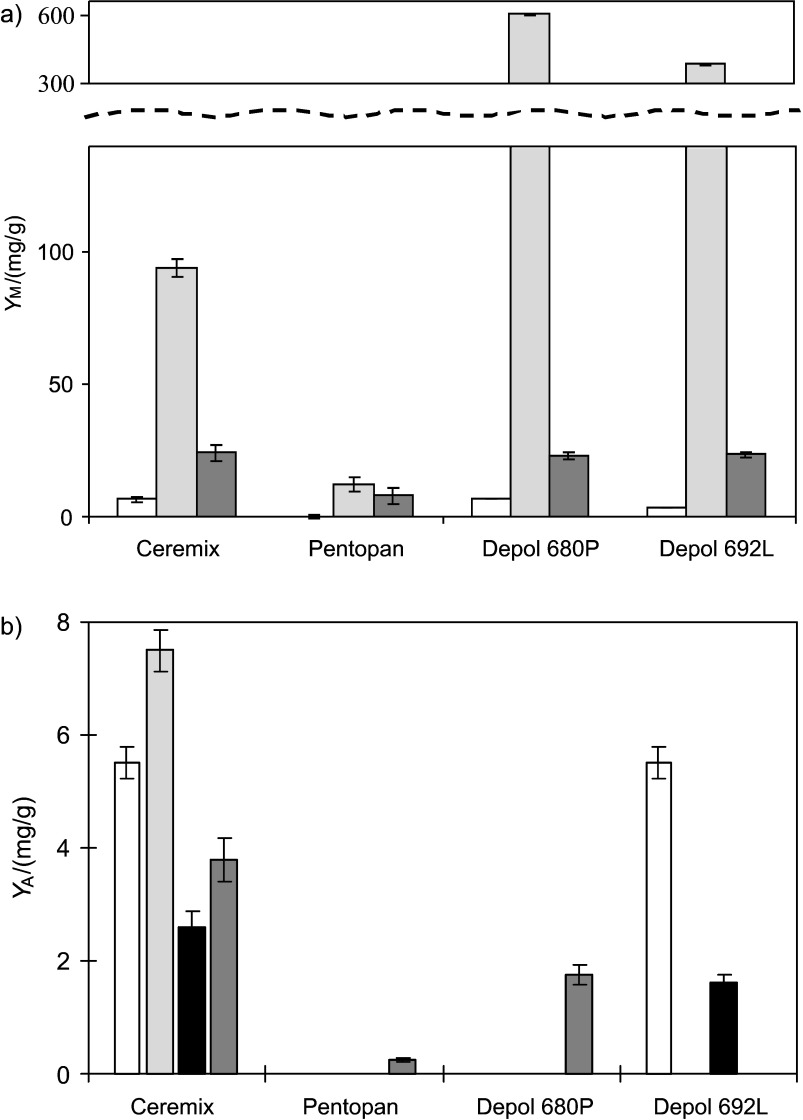
All tested enzyme complexes were capable of degrading rye AX main chain, releasing various monosaccharides and AXOS. d-Arabinose (Ara), d-xylose (Xyl) and d-glucose (Glc) were determined as a result of full AX and starch degradation (Fig. 1a). The results showed that Depol 680P and Depol 692L gave the highest yields of Glc (602.0 and 382.2 mg/g, respectively) and Xyl (23.2 and 23.4 mg/g). Thermomyces lanuginosus-derived xylanase Pentopan Mono BG had the highest activity, but unexpectedly poor release of Xyl, which can be explained by this xylanase assignation to family GH11. According to the results of other authors, a GH11 family xylanase needs the presence of other xylanolytic enzymes to act effectively on AX for complete degradation of polymeric chain (23, 24). Kormelink et al. (25) reported that pure GH10 family endo-1,4-β-d-xylanases (from Aspergillus awamori) converted up to 72% of the wheat flour AX into mono-, di- and oligosaccharides with the degree of polimerisation (DP) of 3–10, whereas endo-1,4-β-d-xylanases from GH11 family hydrolysed only 50% of AX into oligosaccharides with DP=5–10. Shorter AXOS were also formed by GH10 family endo-1,4-β-d-xylanases, compared to those produced by GH11 family endo-1,4-β-d-xylanases (7, 26).
Fig. 1.
Yields of: a) monosaccharides (YM) (white column – arabinose, light gray column – glucose, and black column - xylose), and b) arabinoxylooligosaccharides (YA) (white column - A2XX, light gray column - A2,3X, dark gray column - A2,3XX, and black column - A3X) in enzymatically treated extruded rye
Analysis showed that most of the tested enzyme preparations formed the following AXOS: singly substituted (position C2) arabinoxylotriose (A2XX), singly substituted (position C3) arabinoxylobiose (A3X) and doubly substituted (positions C2 and C3) arabinoxylotriose (A2,3XX). Highest yield of A2XX (5.5 mg/g), A2,3X (7.5 mg/g), A2,3XX (2.6 mg/g) and A3X (3.8 mg/g) was determined in extruded rye samples treated with Ceremix (Fig. 1b). Depol 692L released A2XX (5.5 mg/g) and A2,3XX (1.6 mg/g). Other enzyme complexes were not effective in releasing these AXOS. Therefore, H. insolens- and B. licheniformis-derived enzyme preparation Ceremix was chosen for production of higher value non-alcoholic cereal-based beverage.
Higher value of cereal-based products is related to XOS and AXOS, which according to other authors have shown promising prebiotic properties (27, 28). However, linear oligosaccharides may be fermented rapidly in the colon and thus those with more complex carbohydrate structures are currently studied to find more slowly fermenting prebiotics (29, 30). Van Crayeveld et al. (31) determined that AXOS with DP≤3 caused increased colonic acetate and butyric concentrations and increased the amount of bifidobacteria. However, little information is available on which AXOS would be the most beneficial, because it is complicated to obtain enough purified and well-defined AXOS preparations.
Saccharide profiles in fermented rye mash
Lactic acid bacteria P. pentosaceus and L. sakei and baker’s yeast were used for preparation of a non-alcoholic beverage from extruded rye. Different enzymatic treatments were tested for extruded rye mash preparation: (i) with enzyme composition of α-amylase from A. oryzae, glucoamylase from A. niger and xylanolytic enzyme complex from H. insolens and B. licheniformis (as the best rye AX-degrading enzyme preparation), and (ii) only with xylanolytic enzyme complex. Control mash was prepared from non-enzymatically treated extruded rye. Efficiency of fermentation process was evaluated based on the changes in pH and TTA, saccharide mass fraction as well as the amount of ethanol and some volatile compounds.
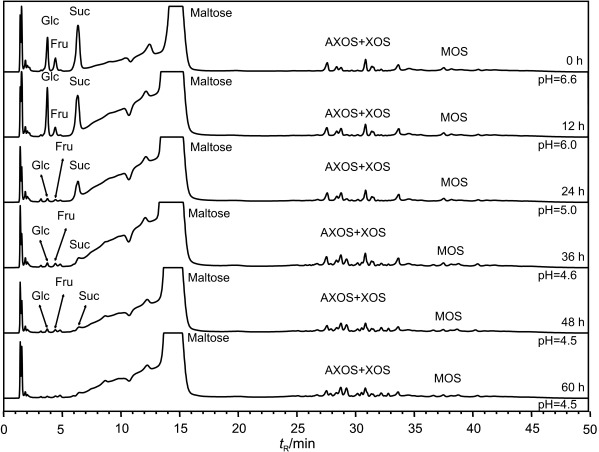
Changes in saccharide mass fraction during fermentation were analysed by HPAEC every 12 h (Fig. 2). The consumption rate of Glc and sucrose (Suc) after 24 h of fermentation showed that the LAB used Glc first as the main substrate, and only when the mass fraction of this monosaccharide in fermentation medium was low, Suc was used (Table 1). In non-enzymatically treated mash, the LAB decreased the mass fraction of Glc by 62.1% (P. pentosaceus) and 69.0% (L. sakei); additionally, Suc mass fraction was decreased by 62.3 and 61.1%, respectively. After treatment with xylanolytic enzyme complex, P. pentosaceus and L. sakei consumed 88.5 and 75.7% of Glc, respectively, and Suc mass fraction remained intact. In the samples treated with amylolytic and xylanolytic enzyme complexes, Glc mass fraction decreased only by 19.3% (P. pentosaceus) and 5.2% (L. sakei), the amount of Suc was not changed. The mass fractions of Ara (10.9–15.2 mg/g) and Xyl (8.1–13.7 mg/g) were almost similar in all enzymatically treated samples fermented with L. sakei and P. pentosaceus and remained unchanged until the end of fermentation.
Fig. 2.
HPAEC elution profiles of the mono- and oligosaccharides, obtained after fermentation of extruded rye by Pediococcus pentosaceus KTU05-10. Glc=glucose, Fru=fructose, Suc=sucrose, AXOS=arabinoxylooligosaccharides, XOS=xylooligosaccharides, MOS=maltooligosaccharides, tR=retention time
Table 1. Mass fraction of saccharides determined in extruded rye mash during fermentation with Pediococcus pentosaceus and Lactobacillus sakei.
| t(fermentation)/h | w(mono-/disaccharides)/(mg/g) | w(oligosaccharides)/(mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Xyl | Ara | Glc | Suc | XOS | AXOS | MOS | ||
| P. pentosaceus | ||||||||
| Amylolytic and xylanolytic enzyme complex | ||||||||
| 0 | 8.2±0.3 | 13.8±0.4 | 238.6±7.3 | 8.7±0.2 | 52.7±1.6 | 17.2±0.5 | 364.7±9.1 | |
| 24 | 8.3±0.3 | 13.8±0.4 | 186.4±5.8 | 8.7±0.3 | 53.5±1.6 | 16.9±0.5 | 349.7±9.0 | |
| 48 | 8.1±0.2 | 13.1±0.4 | 150.4±4.7 | 7.8±0.2 | 51.9±1.6 | 17.1±0.5 | 345.8±9.1 | |
| Xylanolytic enzyme complex | ||||||||
| 0 | 12.0±0.4 | 12.5±0.4 | 128.6±3.9 | 7.9±0.2 | 53.6±1.6 | 16.8±0.5 | 362.2±9.2 | |
| 24 | 9.4±0.3 | 13.5±0.4 | 14.8±0.4 | 7.8±0.2 | 53.7±1.6 | 17.5±0.5 | 365.6±9.3 | |
| 48 | 9.4±0.3 | 10.9±0.3 | 4.0±0.1 | 8.3±0.2 | 52.4±1.6 | 17.7±0.5 | 378.7±8.9 | |
| Without enzymes | ||||||||
| 0 | n.d. | n.d. | 12.6±0.1 | 8.1±0.3 | 0.65±0.0 | 2.3±0.0 | 319.0±9.1 | |
| 24 | n.d. | n.d. | 4.8±0.0 | 3.4±0.2 | 0.69±0.0 | 2.9±0.0 | 310.7±8.8 | |
| 48 | n.d. | n.d. | 2.4±0.0 | 1.5±0.2 | 0.64±0.0 | 3.1±0.0 | 311.8±8.9 | |
| L. sakei | ||||||||
| Amylolytic and xylanolytic enzyme complex | ||||||||
| 0 | 13.7±0.4 | 11.5±0.3 | 253.8±7.5 | 7.3±0.3 | 54.1±1.6 | 18.0±0.5 | 352.4±8.7 | |
| 24 | 8.5±0.3 | 14.4±0.4 | 204.3±6.1 | 7.3±0.3 | 53.9±1.6 | 17.6±0.6 | 353.2±9.2 | |
| 48 | 8.9±0.3 | 15.2±0.5 | 179.0±5.6 | 7.1±0.3 | 53.5±1.6 | 17.3±0.6 | 350.6±9.1 | |
| Xylanolytic enzyme complex | ||||||||
| 0 | 13.7±0.4 | 13.4±0.4 | 103.1±3.1 | 6.4±0.2 | 53.6±1.6 | 16.6±0.5 | 339.5±8.5 | |
| 24 | 8.5±0.3 | 14.6±0.4 | 25.1±0.6 | 6.5±0.2 | 52.7±1.6 | 15.9±0.5 | 346.7±9.2 | |
| 48 | 8.4±0.3 | 14.9±0.5 | 7.2±0.2 | 4.3±0.2 | 53.4±1.6 | 16.5±0.5 | 345.3±9.4 | |
| Without enzymes | ||||||||
| 0 | n.d. | n.d. | 12.9±0.1 | 8.4±0.3 | 1.3±0.0 | 3.8±0.0 | 312.9±8.3 | |
| 24 | n.d. | n.d. | 4.0±0.0 | 3.6±0.2 | 1.2±0.0 | 3.6±0.0 | 306.2±8.8 | |
| 48 | n.d. | n.d. | 2.0±0.0 | 1.3±0.2 | 1.2±0.0 | 4.1±0.0 | 299.8±8.7 | |
Xyl=xylose, Ara=arabinose, Glc=glucose, Suc=sucrose, XOS=xylooligosaccharides, XOS=arabinoxylooligosaccharides, MOS=maltooligosaccharides, n.d.=not detected
No significant changes (p>0.05) of XOS and AXOS in enzymatically treated rye mash were detected during fermentation (Table 1). The following XOS were identified: xylobiose (X2), xylotriose (X3), xylopentose (X5) and xylohexose (X6), and their mass fractions were in the range of 6.5–6.8, 2.3–2.5, 29.9–31.5 and 4.4–4.5 mg/g, respectively (data not shown). The identified AXOS were determined in similar mass fractions (in mg/g): A2XX 2.6–2.8, A2,3XX 2.5–2.7, A2,3X 6.9–7.3, and A3X 4.5–4.8 (data not shown).
Influence of microorganisms on beverage quality
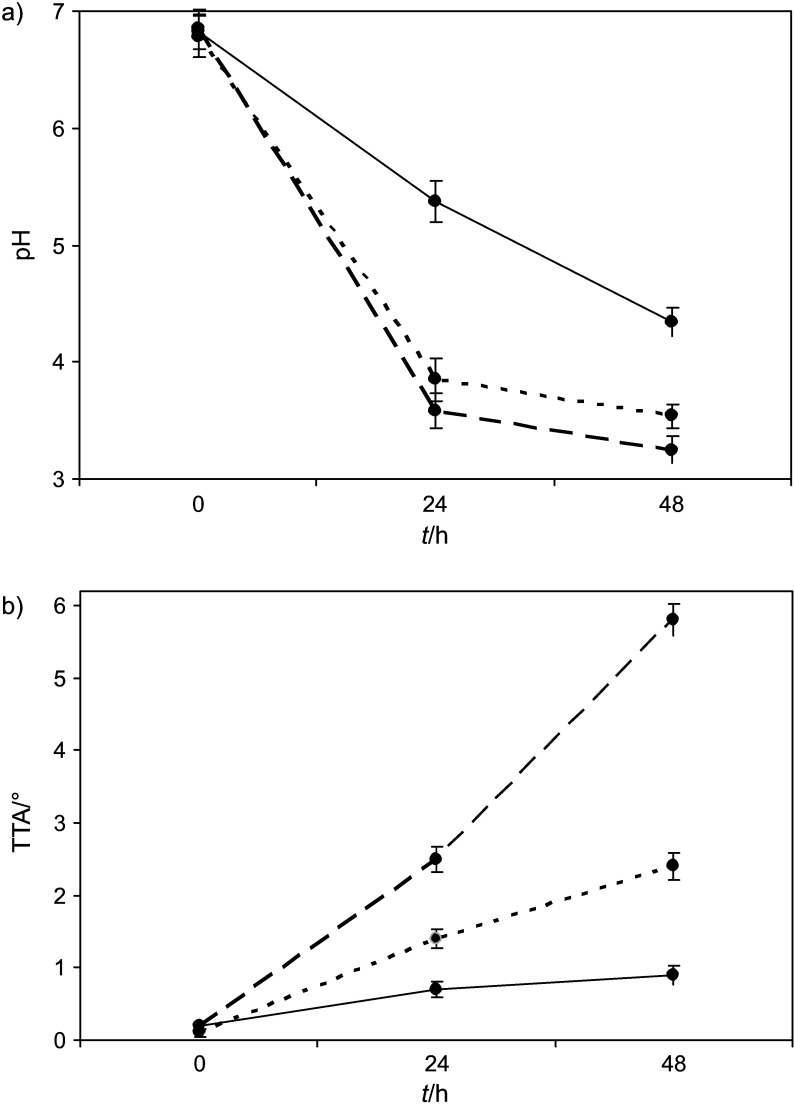
The changes in quality parameters (pH and TTA) of beverages from enzymatically treated (with amylolytic and xylanolytic enzyme complex) extruded rye during fermentation by LAB or yeast are presented in Fig. 3. It was determined that pH of beverages fermented by LAB reached lower values (3.25–3.54) compared to yeast-fermented beverage (4.34). On the other hand, the TTA increased during fermentation, and higher amount of organic acids was detected in LAB-fermented beverages (2.4–5.8 °) than in the yeast-fermented ones (0.9 °). Among LAB, P. pentosaceus produced twice more organic acids than L. sakei. According to the pH and TTA values, the increase of acidity was almost the same as after 24 h. Small changes in pH and TTA values after 48 h of LAB fermentation as compared to 24 h showed that beverage fermentation using LAB can be completed within 24 h.
Fig. 3.
Changes of: a) pH and b) titratable acidity (TTA) in beverages from enzymatically treated (with amylolytic and xylanolytic enzyme complex) extruded rye during fermentation with Pediococcus pentosaceus KTU05-10 (dashed line), Lactobacillus sakei KTU05-6 (dotted line) and Saccharomyces cerevisiae (full line)
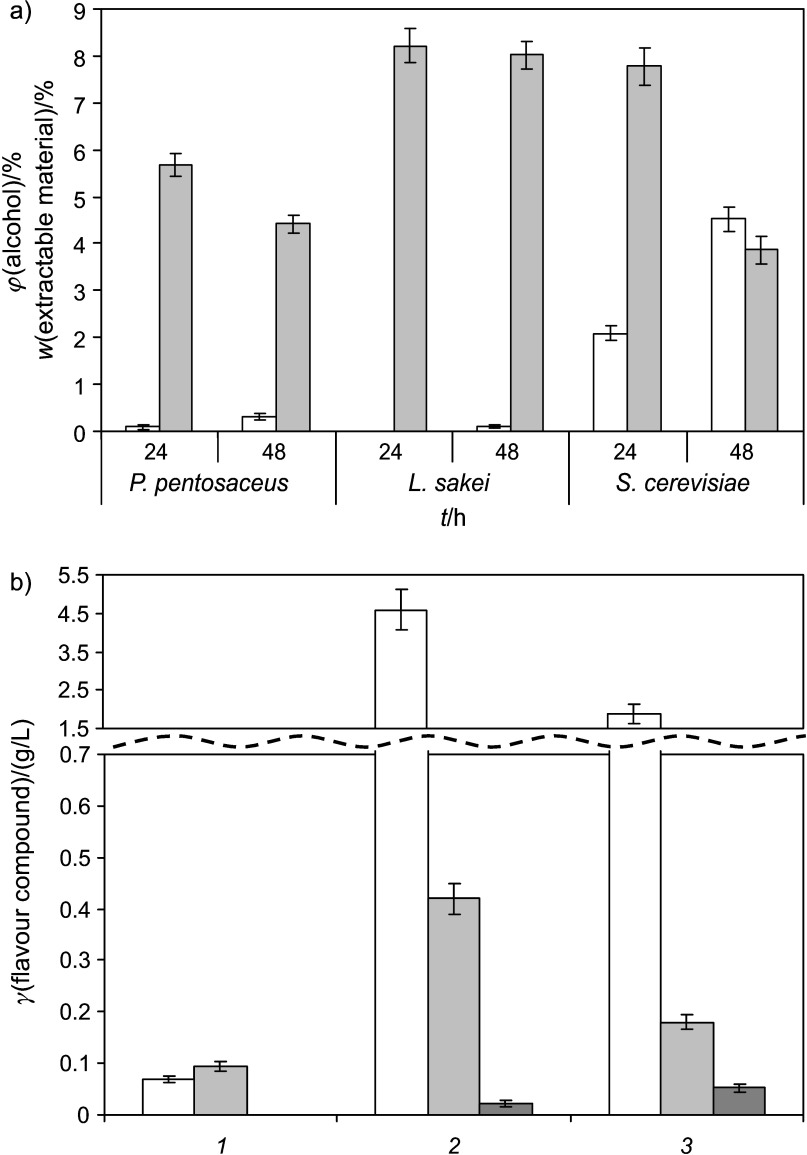
Fermentation of traditional home-made kvass by yeast usually provides from 3 to 4% (by volume) of alcohol in the end product, but in non-alcoholic fermented beverage it should not exceed 1.2% (by volume). The replacement of yeast by LAB allowed decreasing alcohol content in the beverage to an appropriate amount and fulfilling this requirement for non-alcoholic beverages. Determination of alcohol content (Fig. 4a) showed that both LAB produced some amount of alcohol (0.1–0.3%), but it did not exceed the limits for non-alcoholic beverages, while yeast produced 4.5% of alcohol.
Fig. 4.
Content of: a) white column - alcohol and light gray column - extractable materials, and b) flavour compounds (white column - lactic acid, light gray column - acetic acid and black column - methyl acetate) in fermented beverage from enzymatically treated extruded rye
LAB fermentation as compared to yeast provided more flavour compounds that participate in the formation of product aroma and taste (Fig. 4b). The main product of LAB fermentation is lactic acid, but some other compounds, like acetic acid, diacetyl, methylacetate or various esters are released. Comparing both LAB, it was determined that P. pentosaceus produced twice more acetic and lactic acids than L. sakei. Also in samples fermented by both LAB a small amount of methyl acetate was detected, which gives a pleasant aroma of fruits to beverages.
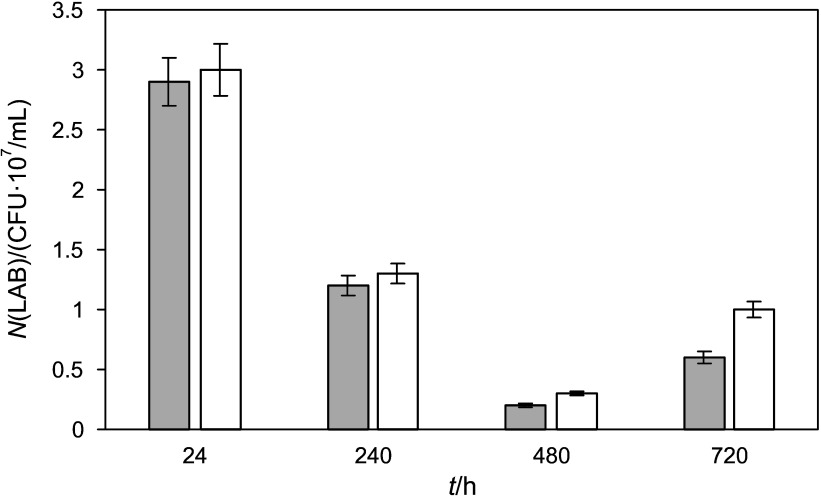
For estimation of LAB antimicrobial properties, lactic acid bacteria cell count and total microorganism cell count were evaluated during beverage storage for 30 days at 0–6 °C (Fig. 5). Analysis showed that after 30 days, viable cells of L. sakei decreased in the beverage more than 4 times (from 2.8·107 to 6.2·106 CFU/mL), but viability of LAB remained high (more than 106 CFU/mL). The use of L. sakei resulted in a reduced total microorganism growth in the beverage during storage and confirmed that this LAB is capable of retarding the growth of extraneous microorganisms.
Fig. 5.
The growth of microorganisms (gray column - total LAB cell count, white column - total microorganism cell count) during storage in a beverage fermented by Lactobacillus sakei
Sensory characteristics and acceptability of beverages
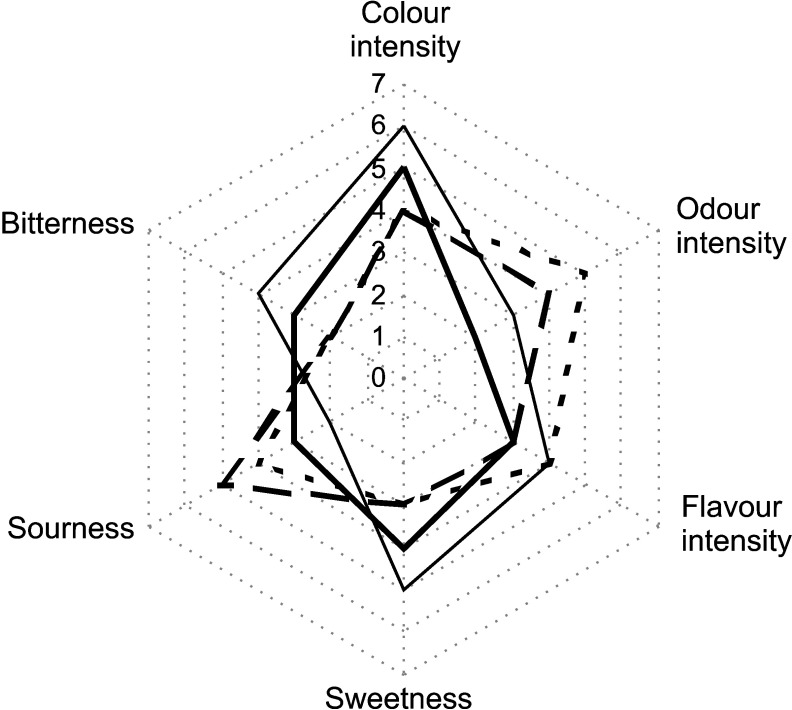
Statistically significant (p≤0.05) differences between commercial kvass made from rye malt extract (control) and beverages made from extruded rye fermented by yeast or LAB were determined in sweetness, sourness, colour and odour intensity (Fig. 6). The use of extruded rye for beverage production increased odour and decreased colour intensity of the product. Furthermore, using extruded raw material for fermentation, intensity of brown colour was lost, compared to the control kvass. Beverages fermented by LAB also changed flavour properties: sweetness and bitterness decreased, while sourness increased.
Fig. 6.
Descriptive sensory analysis profile of commercial kvass (control) (thin line) and non-alcoholic beverages from extruded rye fermented by Saccharomyces cerevisiae (thick line), Pediococcus pentosaceus KTU05-10 (em dash line) and Lactobacillus sakei KTU05-6 (en dash line)
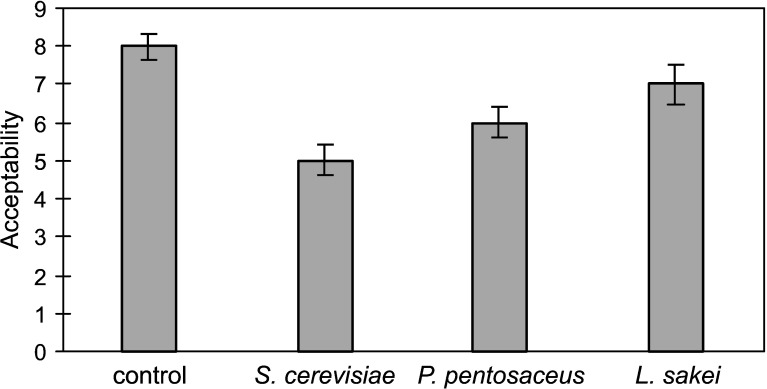
Acceptability (Fig. 7) of beverages fermented by LAB was lower (p≤0.05) compared to commercial kvass, but it was higher (p≤0.05) than of beverage fermented by yeast. Comparing both LAB, higher acceptability was determined of the beverage fermented by L. sakei than by P. pentosaceus. Assessors preferred L. sakei-fermented beverage due to its higher flavour and odour intensity and lower sourness.
Fig. 7.
Acceptability on a hedonic scale of commercial kvass (control) and non-alcoholic beverages from extruded rye fermented by Saccharomyces cerevisiae, Pediococcus pentosaceus KTU05-10 and Lactobacillus sakei KTU05-6
Conclusions
Extruded rye, xylanolytic enzymes and LAB can be used for production of novel, safe and higher value (with increased amount of oligosaccharides) non-alcoholic beverages. Using the selected xylanolytic enzyme complex Ceremix (Humicola insolens and Bacillus licheniformis) the yield of AXOS and XOS in beverage mash was increased by 300 and 1100 mg/L, respectively, which remained intact during fermentation by LAB. Beverages fermented by the LAB (Pediococcus pentosaceus and Lactobacillus sakei) with antimicrobial activity had lower pH values (3.3–3.5) and higher TTA values (2.4–5.8°), higher concentrations of volatile compounds (0.22–0.45 g/L) and less alcohol (0.1–0.3% by volume) than the beverage fermented by yeast (pH=4.3, TTA=0.9°, γ(volatile compounds)=0.09 g/L and ϕ(alcohol)=4.5%). The acceptability of the beverage from extruded rye fermented by L. sakei was higher than of the yeast-fermented beverages and close to the acceptability of commercial kvass made from rye malt extract.
Acknowledgement
The research was partly funded by a grant of the Research Council of Lithuania. The authors thank Novozymes A/S (Denmark), Biocatalyst Ltd. (UK) and DSM (the Netherlands) for providing the enzyme preparations.
References
- 1.Blandino A. AL-Aseeri ME, Pandiella SS, Cantero D, Webb C. Cereal-based fermented foods and beverages. Food Res Int. 2003;36:527–43. 10.1016/S0963-9969(03)00009-7 [DOI] [Google Scholar]
- 2.Dlusskaya E, Jänsch A, Schwab C, Gänzle MG. Microbial and chemical analysis of a kvass fermentation. Eur Food Res Technol. 2008;227:261–6. 10.1007/s00217-007-0719-4 [DOI] [Google Scholar]
- 3.Dalié DKD, Deschamps AM, Richard-Forget F. Lactic acid bacteria – potential for control of mould growth and mycotoxins: a review. Food Contr. 2010;21:370–80. 10.1016/j.foodcont.2009.07.011 [DOI] [Google Scholar]
- 4.Saulnier L, Sado PE, Branlard G, Charmet G, Guillon F. Wheat arabinoxylans: exploiting variation in amount and composition to develop enhanced varieties. J Cereal Sci. 2007;46:261–81. 10.1016/j.jcs.2007.06.014 [DOI] [Google Scholar]
- 5.Kamal-Eldin A, Lćrke HN, Bach Knudsen KE, Lampi AM, Piironen V, Adlercreutz H, et al. Physical, microscopic and chemical characterisation of industrial rye and wheat brans from the Nordic countries. Food Nutr Res. 2009;53: 10.3402/fnr.v52i0.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75. 10.1079/NRR200479 [DOI] [PubMed] [Google Scholar]
- 7.Van Laere KMJ, Hartemink R, Bosveld M, Schols HA, Voragen AGJ. Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. J Agric Food Chem. 2000;48:1644–52. 10.1021/jf990519i [DOI] [PubMed] [Google Scholar]
- 8.Grootaert C, Delcour JA, Courtin CM, Broekaert WF, Verstraete W, Van de Wiele T. Microbial metabolism and prebiotic potency of arabinoxylan oligosaccharides in the human intestine. Trends Food Sci Technol. 2007;18:64–71. 10.1016/j.tifs.2006.08.004 [DOI] [Google Scholar]
- 9.Pastell H, Westermann P, Meyer AS, Tuomainen P, Tenkanen M. In vitro fermentation of arabinoxylan-derived carbohydrates by bifidobacteria and mixed fecal microbiota. J Agric Food Chem. 2009;57:8598–606. 10.1021/jf901397b [DOI] [PubMed] [Google Scholar]
- 10.Holguín-Acuńa AL, Carvajal-Millán E, Santana-Rodríguez V, Rascón-Chu A, Márquez-Escalante JA, de León-Renova NEP, et al. Maize bran/oat flour extruded breakfast cereal: a novel source of complex polysaccharides and an antioxidant. Food Chem. 2008;111:654–7. 10.1016/j.foodchem.2008.04.034 [DOI] [Google Scholar]
- 11.Patil RT, Berrios JDJ, Tang J, Swanson BG. Evaluation of methods for expansion properties of legume extrudates. Appl Eng Agric. 2007;23:777–83. 10.13031/2013.24044 [DOI] [Google Scholar]
- 12.Singh S, Gamlath S, Wakeling L. Nutritional aspects of food extrusion: a review. Int J Food Sci Technol. 2007;42:916–29. 10.1111/j.1365-2621.2006.01309.x [DOI] [Google Scholar]
- 13.Kahlon TS, Edwards RH, Chow FI. Effect of extrusion on hypocholesterolemic properties of rice, oat, corn, and wheat bran diets in hamsters. Cereal Chem. 1998;75:897–903. 10.1094/CCHEM.1998.75.6.897 [DOI] [Google Scholar]
- 14.Vasanthan T, Gaosong J, Yeung J, Li J. Dietary fiber profile of barley flour as affected by extrusion cooking. Food Chem. 2002;77:35–40. http://dx.doi.org/S0308-8146(01)00318-1 10.1016/S0308-8146(01)00318-1 [DOI] [Google Scholar]
- 15.Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–70. 10.1016/0168-1656(92)90074-J [DOI] [Google Scholar]
- 16.Zurbriggen B, Bailey MJ, Pentillä ME, Poutanen K, Linko M. Pilot scale production of heterologous Trichoderma reesei cellulose in Saccharomyces cerevisiae. J Biotechnol. 1990;13:267–78. 10.1016/0168-1656(90)90075-M [DOI] [PubMed] [Google Scholar]
- 17.Poutanen K, Rättö M, Puls J, Viikari L. Evaluation of different microbial xylanolytic systems. J Biotechnol. 1987;6:49–60. 10.1016/0168-1656(87)90045-9 [DOI] [Google Scholar]
- 18.Poutanen K, Puls J. Characteristics of Trichoderma reesei β-xylosidase and its use in the hydrolysis of solubilized xylans. Appl Microbiol Biotechnol. 1988;28:425–32. 10.1007/BF00268208 [DOI] [Google Scholar]
- 19.Cizeikiene D, Juodeikiene G, Paskevicius A, Bartkiene E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Contr. 2013;31:539–45. 10.1016/j.foodcont.2012.12.004 [DOI] [Google Scholar]
- 20.Rantanen H, Virkki L, Tuomainen P, Kabel M, Schols H, Tenkanen M. Preparation of arabinoxylobiose from rye xylan using family 10 Aspergillus aculeatus endo-1,4-β-D-xylanase. Carbohydr Polym. 2007;68:350–9. 10.1016/j.carbpol.2006.11.022 [DOI] [Google Scholar]
- 21.Pastell H, Tuomainen P, Virkki L, Tenkanen M. Step-wise enzymatic preparation and structural characterization of singly and doubly substituted arabinoxylo-oligosaccharides with non-reducing end terminal branches. Carbohydr Res. 2008;343:3049–57. 10.1016/j.carres.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 22.ISO 4833-2:2013. Microbiology of the food chain – Horizontal method for the enumeration of microorganisms – Part 2: Colony count at 30 degrees C by the surface plating technique. Geneva, Switzerland: International Organization for Standardization (ISO); 2013.
- 23.Pell G, Taylor EJ, Gloster TM, Turkenburg JP, Fontes CMGA, Ferreira LMA, et al. The mechanisms by which family 10 glycoside hydrolases bind decorated substrates. J Biol Chem. 2004;279:9597–605. 10.1074/jbc.M312278200 [DOI] [PubMed] [Google Scholar]
- 24.Vardakou M, Katapodis P, Samiotaki M, Kekos D, Panayotou G, Christakopoulos P. Mode of action of family 10 and 11 endoxylanases on water unextractable arabinoxylan. Int J Biol Macromol. 2003;33:129–34. 10.1016/S0141-8130(03)00077-1 [DOI] [PubMed] [Google Scholar]
- 25.Kormelink FJM, Gruppen H, Viëtor RJ, Voragen AGJ. Mode of action of the xylan-degrading enzymes from Aspergillus awamori on alkali-extractable cereal arabinoxylans. Carbohydr Res. 1993;249:355–67. 10.1016/0008-6215(93)84100-K [DOI] [PubMed] [Google Scholar]
- 26.Puchart V, Biely P. Simultaneous production of endo-β-1,4-xylanase and branched xylooligosaccharides by Thermomyces lanuginosus. J Biotechnol. 2008;137:34–43. 10.1016/j.jbiotec.2008.07.1789 [DOI] [PubMed] [Google Scholar]
- 27.Grootaert C, Van den Abbeele P, Marzorati M, Broekaert WF, Courtin CM, Delcour JA, et al. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol. 2009;69:231–42. 10.1111/j.1574-6941.2009.00712.x [DOI] [PubMed] [Google Scholar]
- 28.Gullón P, González-Muńoz MJ, van Gool MP, Schols HA, Hirsch J, Ebringerová A, et al. Production, refining, structural characterization and fermentability of rice husk xylooligosaccharides. J Agric Food Chem. 2010;58:3632–41. 10.1021/jf904508g [DOI] [PubMed] [Google Scholar]
- 29.Kabel MA, Kórtenoeven L, Schols HA, Voragen AGJ. In vitro fermentability of differently substituted xylo-oligosaccharides. J Agric Food Chem. 2002;50:6205–10. 10.1021/jf020220r [DOI] [PubMed] [Google Scholar]
- 30.Moura P, Barata R, Carvalheiro F, Gírio F, Loureiro-Dias MC, Esteves MP. In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bififdobacterrium and Lactobacillus strains. LWT –. Food Sci Technol (Campinas). 2007;40:963–72. 10.1016/j.lwt.2006.07.013 [DOI] [Google Scholar]
- 31.Van Crayeveld V, Swennen K, Dornez E, Van de Wiele T, Marzorati M, Verstraete W, et al. Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J Nutr. 2008;138:2348–55. 10.3945/jn.108.094367 [DOI] [PubMed] [Google Scholar]