Abstract
Meiotic recombination establishes connections between homologous chromosomes to promote segregation. Hemizygous regions of sex chromosomes have no homologous chromosome to recombine with, yet must be transmitted through meiosis. An extreme case of hemizygosity exists in the genus Caenorhabditis, where males have a single X chromosome that completely lacks a homologous partner. To determine whether similar strategies have evolved to accommodate hemizygosity of the X during male meiosis in Caenorhabditis with distinct modes of sexual reproduction, we examined induction and processing of meiotic double strand breaks (DSBs) in androdioecious (hermaphrodite/male) Caenorhabditis elegans and C. briggsae, and gonochoristic (female/male) C. remanei and C. brenneri. Analysis of the recombinase RAD-51 suggests more meiotic DSBs are induced in gonochoristic vs. androdioecious species. However, in late prophase in all species, chromosome pairs are restructured into bivalents around a single axis, suggesting that the holocentric nature of Caenorhabditis chromosomes dictates a single crossover per bivalent regardless of the number of DSBs induced. Interestingly, RAD-51 foci were readily observed on the X chromosome of androdioecious male germ cells, while very few were detected in gonochoristic male germ cells. As in C. elegans, the X chromosome in C. briggsae male germ cells undergoes transient pseudosynapsis and flexibility in DSB repair pathway choice. In contrast, in C. remanei and C. brenneri male germ cells, the X chromosome does not undergo pseudosynapsis and appears refractory to SPO-11-induced breaks. Together our results suggest that distinct strategies have evolved to accommodate sex chromosome hemizygosity during meiosis in closely related Caenorhabditis species.
Keywords: double strand breaks (DSBs), meiosis, RAD-51, sex chromosomes, synapsis, Genetics of Sex
MEIOSIS is essential for sexual reproduction and involves the precise halving of the genome for packaging into gametes. Meiosis relies on homology between maternal and paternal chromosomes to form physical connections for accurate chromosome segregation (reviewed in Zickler and Kleckner 2015). While the overall meiotic program is similar between the sexes, meiosis is sexually dimorphic with respect to timing, the recombination landscape, and mechanisms of meiotic chromosome segregation (reviewed in Morelli and Cohen 2005). Additionally, the presence of differentiated sex chromosomes in the heterogametic sex contributes to differences in female and male meiosis. Heterogametic sex chromosomes have limited or no homology, but as with the autosomes must be accurately transmitted to the next generation.
In meiotic cells, both autosomes and sex chromosomes exists in an environment where recombination is actively engaged to ensure the formation of crossovers. Meiotic recombination is initiated by DNA double strand breaks (DSBs) mediated by the conserved topoisomerase Spo11 and associated proteins (Keeney et al. 1997; Dernburg et al. 1998; Cole et al. 2010; Rosu et al. 2013; Stamper et al. 2013). Meiotic DSBs are processed to promote homologous recombination (HR). Central to HR, is break resection (Penkner et al. 2007; Garcia et al. 2011; Lemmens et al. 2013; Yin and Smolikove 2013) and loading of recA recombinases RAD51/DMC1, which mediate strand invasion (Bishop et al. 1992; Alpi et al. 2003; Brown and Bishop 2015). A subset of processed breaks is converted into crossovers, which require unique molecular machinery and provide the physical connection between homologs. Recent work has established that crossover formation is exquisitely controlled and involves both repair in the context of the specialized meiotic chromosome structure and regulatory mechanisms to monitor DSB formation, processing, and ultimately, crossover formation (Shinohara et al. 2008; Rosu et al. 2011, 2013; Stamper et al. 2013; Yu et al. 2016).
Our understanding of how meiosis in general, and sex chromosomes specifically, is differentially regulated in the sexes is limited to the small number of model organisms where it has been examined mechanistically, most notably in Mus musculus, Drosophila melanogaster, and C. elegans. Each of these organisms uses a different strategy for accommodating sex chromosomes during meiosis, suggesting that these strategies arose independently in concert with the emergence of sex chromosomes in the different lineages. For example, in mice the obligate crossover at the homologous pseudoautosomal region (PAR) between the X and the Y requires a specific isoform of Spo11 and specialized chromosome structure (Kauppi et al. 2011). On the other hand, in Drosophila males, meiosis is achiasmatic and thus does not rely on meiotic recombination at all; however, pairing and segregation of the X–Y sex chromosomes is dependent on ribosomal DNA homology between the chromosomes (McKee 1996).
The nematode, C. elegans, represents an extreme example of hemizygosity as the X chromosome of males completely lacks a pairing partner. Further, as in mammals, the X chromosome is transcriptionally silenced by meiotic sex chromosome inactivation (Kelly et al. 2002; Reuben and Lin 2002; Bean et al. 2004; Checchi and Engebrecht 2011). We previously reported that the X chromosome of C. elegans males undergoes a brief period of pseudosynapsis, [i.e., apparent synapsis that does not necessarily constitute full alignment or contain all components of the synaptonemal complex (SC)] concurrent with the formation of SPO-11-dependent DSBs (Checchi et al. 2014). However, the X chromosome is refractory to DSB feedback mechanisms, which control and regulate recombination on the autosomes. Further, unlike autosomes, X-specific breaks can be repaired in the absence of HR (Checchi et al. 2014), suggesting alternative DSB repair pathways can be engaged. C. elegans is one member of a large and diverse family of nematodes, with distinct modes of sexual reproduction and occupying wide ecological niches (Kiontke and Fitch 2005; Kiontke et al. 2011). Whether the same strategies are used in other Caenorhabditis species is not known. Here we compare meiotic DSB patterns genomewide and on the X chromosome in Caenorhabditis species that are hermaphroditic/male or obligate female/male. We find strikingly different strategies for sex chromosome transmission among these closely related Caenorhabditis species, suggesting a remarkable degree of divergence in the behavior of hemizygous sex chromosomes during meiosis even within the same lineage.
Materials and Methods
Genetics
Strains were maintained at 20° unless otherwise noted. C. elegans var. Bristol (N2), C. briggsae (AF16), C. remanei (SB146), C. brenneri (CB5161), C. nigoni (JU1325), and C. tropicalis (JU1373) were used as the wild-type strain for each respective species. C. briggsae and C. remanei RNA interference (RNAi) sensitive by feeding strains JU1018:mfIs42[Cel-sid-2 + Cel-myo-2::DsRed] and JU1184:mfEx34[Cel-sid-2 + Cel-myo-2::DsRed] were generated by Nuez and Felix (2012). All experiments were performed on animals maintained at a ratio of one female/hermaphrodite to three males. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources.
Embryonic lethality was determined by counting eggs and hatched larvae 24 hr after removing adult and calculating percentage as eggs/eggs + larvae.
Cytological analysis
Immunostaining of germ lines was performed as described (Jaramillo-Lambert et al. 2007). The following primary antibodies were purchased and used at the indicated dilutions: mouse anti-histone H3K4me2 (1:500; catalog no. 05-1338) and rabbit anti-pH 3S10 (1:500; catalog no. 06-570) (Millipore, Temecula, CA); rabbit anti-RAD-51 (1:10,000; catalog no. 29480002) (Novus Biologicals, Littleton, CO); and mouse anti-nuclear pore complex proteins (Mab414) (1:250; catalog no. ab24609) (Abcam, Cambridge, MA). Rabbit anti-AIR-2 (1:500) was generously provided by Jill Schumacher (University of Texas) and rat anti-RAD-51 (1:100) was generously provided by Anne Villeneuve (Stanford University). Goat anti-SYP-1 (1:250) and rabbit anti-SYP-2 (1:250) were generous gifts from Sarit Smolikove (University of Iowa). The following secondary antibodies from Life Technologies were all used at 1:500 dilutions: Alexa Fluor 594 donkey anti-mouse IgG, Alexa Fluor 488 goat anti-mouse IgG, Alexa Fluor 596 donkey anti-rabbit IgG, Alexa Fluor 488 donkey anti-rabbit IgG, Alexa Fluor 488 donkey anti-goat IgG, and Alexa Fluor 555 goat anti-rat IgG. Alexa Fluor 647 goat anti-rabbit was used at 1:250 dilution. DAPI (2 μg/ml; Sigma, St. Louis, MO) was used to counterstain DNA.
Collection of images was performed using an API Delta Vision deconvolution microscope or Nikon Instruments Eclipse Ti-E microscope. A minimum of three germ lines was examined for each condition and experiments were repeated a minimum of three times. Images were deconvolved using Applied Precision SoftWoRx or Huygens imaging analysis software and subsequently processed and analyzed using Fiji (ImageJ) (Wayne Rasband, NIH).
RAD-51 foci were quantified in three germ lines of age-matched hermaphrodites/females or males (24 hr post-L4). A single gonadal arm was scored in hermaphrodites/females. Germ lines were divided by meiotic prophase substage based on morphology and position; transition zone was counted from the first row with three or more crescent-shaped nuclei until the last row with three or more crescent-shaped nuclei, and pachytene was divided into three equal sections for early (EP), mid (MP), and late pachytene (LP). The number of foci per nucleus was scored for each stage. Quantification of RAD-51 foci specific to the X chromosome(s) was scored as foci that localized to the chromosome that lacked the activating mark H3K4me2.
RNAi analysis
RNAi experiments were performed at 20°, using the feeding method (Timmons et al. 2001). C. briggsae or C. remanei L4 hermaphrodites/females were mated with mix-staged males. After 24 hr, hermaphrodites/females with mating plugs were transferred onto RNAi plates. The hermaphrodite/female and male progeny that grew up on the RNAi plates were transferred to a second RNAi plate and the resultant progeny were analyzed for progeny viability as well as by antibody staining of dissected germ lines. Cbr-rad-51, Cbr-rad-54, and Cbr-spo-11 sequences were isolated from AF16 genomic DNA and Cre-rad-51.2 and Cre-spo-11 were isolated from SB146 genomic DNA. The resulting PCR fragments were inserted into the L4440 vector by Gibson assembly. Primers used for cloning are listed in Supplemental Material, Table S1. Cultures were plated onto NGM plates containing 25 µg/ml carbenicillin and 1 mM IPTG and were used within 2 weeks.
Irradiation treatment
For experiments assessing formation of RAD-51 foci following irradiation (IR) treatment, 24 hr post-L4 worms were exposed to 10–30 Gy of γ-irradiation from a 137Cs source; gonads were dissected and fixed for immunofluorescence as above, at 30 min to 8 hr post-IR.
Data and regeant availability
Plasmids generated and complete imaging data sets are available upon request.
Results
Patterns of genomewide RAD-51 are similar in closely related Caenorhabditis species
The spatiotemporal organization of the C. elegans germ line has facilitated cytological analyses of recombination as all stages of meiotic prophase are present and readily visualized within a single animal (Shakes et al. 2009; Lui and Colaiácovo 2013) (Figure 1, A and D). Specifically, the loading and disassembly of the recombinase RAD-51 by immunostaining has served as a proxy for ongoing meiotic DSB repair. SPO-11-dependent RAD-51 foci are first detected in early prophase (transition zone, TZ), peak in EP to MP, and are largely disassembled by LP (Colaiácovo et al. 2003). While the overall pattern of RAD-51 foci is similar in hermaphrodite and male germ lines, RAD-51 foci peak earlier in male germ cells (Jaramillo-Lambert and Engebrecht 2010; Checchi et al. 2014) (Figure 1).
Figure 1.
RAD-51 as a readout for DNA break repair in Caenorhabditis species. (A and D) Diagrams of the spatiotemporal organization of the hermaphrodite (A) and male (D) Caenorhabditis germ lines. Female Caenorhabditis germ lines look the same except that they do not make sperm. (B and E) C. elegans, C. briggsae, C. remanei, and C. brenneri female/hermaphrodite and male pachytene nuclei stained with DAPI (blue), anti-H3K4me2 (green), and RAD-51 (red). (B) Below is the phylogenetic relationship between the species (redrawn from Kiontke et al. 2011). (C and F) Comparison of average number of RAD-51 foci/nucleus at the premeiotic; proliferative zone (PZ), transition zone (TZ), early pachytene (EP), mid pachytene (MP), and late pachytene (LP) for each Caenorhabditis species. A minimum of three germ lines were examined for each species. Error bars = SEM. (G) TZ and EP nuclei stained with DAPI (blue) and anti-RAD-51 (red) from C. briggsae males treated with indicated RNAi. (H) TZ and EP nuclei stained with DAPI (blue) and anti-RAD-51 (red) isolated from C. remanei females treated with Cre-rad-51 dsRNA. (I) Comparison of average number of RAD-51 foci/nucleus during PZ, TZ, EP, MP, and LP for C. nigoni and C. tropicalis males. All bars, 5 µM.
To monitor meiotic DSB repair in Caenorhabditis species, we examined the localization of RAD-51 by immunostaining in germ lines of C. elegans and C. briggsae, two lineages that independently evolved hermaphrodism, and the obligate female/male species, C. remanei and C. brenneri. The organization of the germ line is conserved in these species, allowing for comparison of RAD-51 assembly and disassembly (Figure 1A and Figure S2), although there are differences in numbers of nuclei at the different meiotic prophase substages (Larson et al. 2016). Consequently, we scored the number of RAD-51 foci/nucleus in each zone and compared this number between the species (Figure 1 and Figure S3). We found the overall pattern of RAD-51 loading and removal to be similar between all of the species; very low numbers of RAD-51 foci were observed in the proliferative zone (PZ) and entry into meiosis (TZ) marked an increase in RAD-51 foci, which peaked at EP during oogenesis and at TZ to EP during spermatogenesis (Figure 1, B, C, E, and F and Figure S3, A and B). Further, RAD-51 declined to very low levels by LP in all species, except in C. remanei females, where substantial numbers of RAD-51 were still observed in LP but were reduced by diplotene (Figure 1, B, C, E, and F and Figure S3).
The pattern of RAD-51 appearance and disappearance is consistent with the hypothesis that we are monitoring meiotic DSBs in these other species as has been demonstrated in C. elegans hermaphrodites (Colaiácovo et al. 2003) and males (Jaramillo-Lambert and Engebrecht 2010). To confirm this, we used C. briggsae and C. remanei strains expressing C. elegans SID-2 from an integrated chromosomal array, which makes them susceptible to RNAi by feeding (Nuez and Felix 2012), and grew the corresponding worms in the presence of Cbr-spo-11, Cre-spo-11, Cbr-rad-51, or Cre-rad-51.2 double stranded RNA (dsRNA). Cbr-spo-11(RNAi) and Cbr-rad-51(RNAi) worms grown for two generations in the presence of dsRNA had elevated levels of embryonic lethality (embryonic lethality: Cbr-WT (L4440 control) = 2.7 ± 0.8%; Cbr-spo-11(RNAi) = 39.3 ± 5.8%, P < 0.01; Cbr-rad-51(RNAi) = 99 ± 1.5%, P < 0.0001) and greatly reduced levels of RAD-51 foci within both the C. briggsae hermaphrodite (data not shown) and male germ lines (Figure 1G), indicating that we are specifically monitoring SPO-11-induced meiotic DSBs loaded with RAD-51. For C. remanei, spo-11(RNAi) did not result in a reduction in either progeny viability or RAD-51 foci (data not shown), suggesting that the RNAi was not efficient. On the other hand, Cre-rad-51.2(RNAi) worms had elevated levels of embryonic lethality (embryonic lethality: Cre-WT (L4440 control) = 14.4 ± 5.6% vs. Cre-rad-51.2(RNAi) = 61.8 ± 8.3%, P = 0.0007) and female germ lines had reduced levels of RAD-51 foci (Figure 1H). Together, the observed pattern of RAD-51 foci in the germ line in all of the species along with the demonstrated specificity of the RAD-51 antibody in C. briggsae and C. remanei, suggest that RAD-51 immunostaining can be used as a readout of meiotic DSB repair in Caenorhabditis.
While the overall patterns are similar, the female/male species had significantly more RAD-51 foci throughout meiotic prophase during both oogenesis and spermatogenesis than the hermaphrodite/male species (Figure 1, C and F, compare red and blue lines, and Figure S3 and Figure S4). Consistent with elevated RAD-51 levels being correlated with gonochorism, C. nigoni, a sister species of C. briggsae (Woodruff et al. 2010; Kiontke et al. 2011), also had high numbers of RAD-51 foci, while the other hermaphrodite/male species in the elegans group, C. tropicalis, had low levels of RAD-51 foci (Figure 1I). Thus, female/male species appear to have overall higher numbers of DSBs as monitored by RAD-51.
Conservation of bivalent restructuring suggests a single crossover is formed per chromosome pair in Caenorhabditis
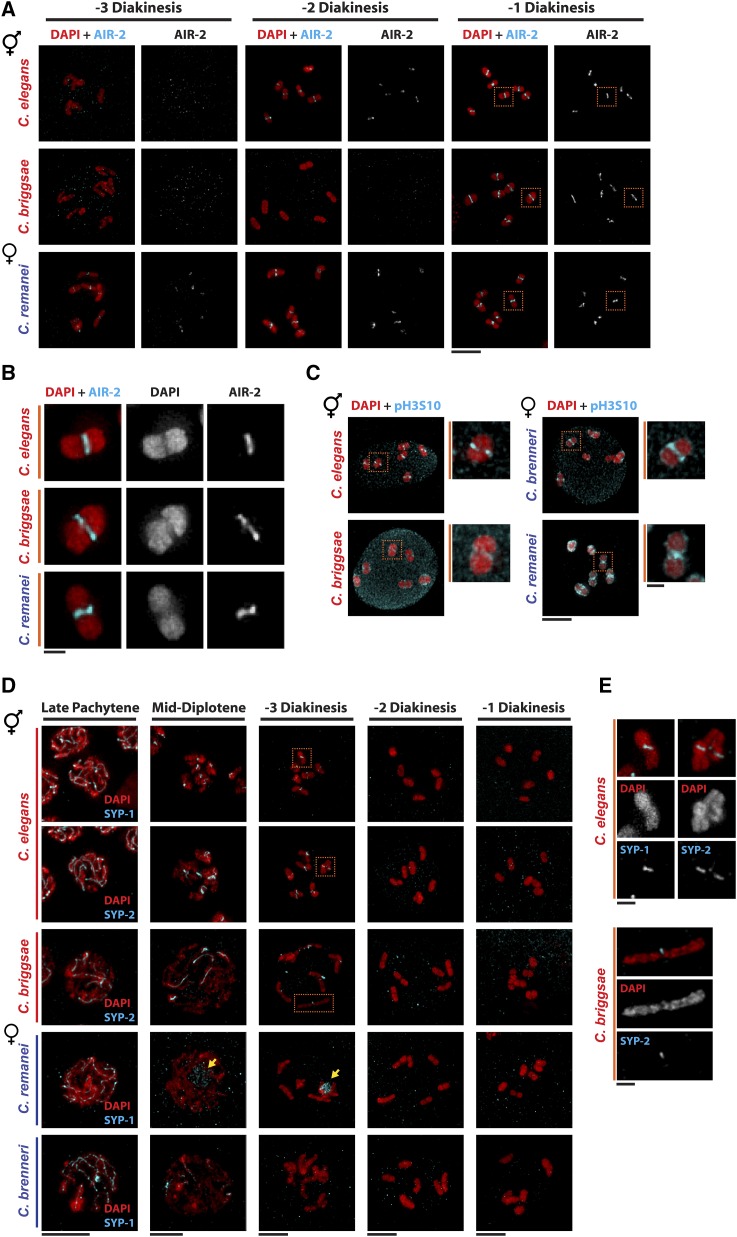
In all organisms where they have been examined, many more meiotic DSBs are induced than will be processed into crossovers (reviewed in Lake and Hawley 2016). In C. elegans, a single, off-centered crossover is formed on most chromosome pairs, presumably due to the holocentric nature of the chromosomes (Albertson et al. 1997; Hillers and Villeneuve 2003; Hammarlund et al. 2005). The position of the crossover dictates the restructuring of the bivalent into a long and short arm for regulated cohesion release and accurate chromosome segregation (Nabeshima et al. 2005; de Carvalho et al. 2008). As the Caenorhabditis genus is holocentric (Coghlan 2005; Zedek and Bures 2012), it is likely that a single crossover is formed even in the female/male species where we observed significantly more RAD-51 foci. Consistent with this, two-lobed bivalents at diakinesis resembled those in C. elegans (Figure 2). To probe the organization of the bivalent, we stained oogenic germ lines from all four species with antibodies directed against Cel-AIR-2 (Aurora B kinase ortholog) (Figure S1B) and examined diakinesis oocytes (−3, −2, −1 position from the spermatheca; Figure 1A). Aurora B is important for regulated cohesin release and specifically marks the short arm of the bivalent (Kaitna et al. 2002; Rogers et al. 2002; de Carvalho et al. 2008). We observed discrete AIR-2 staining at the interface between the bivalent lobes in C. elegans, C. briggsae, and C. remanei, which presumably represent the short arm (Figure 2, A and B). However, the timing of AIR-2 localization at the midbivalent was distinct: in C. elegans, AIR-2 was observed on the short arms beginning in the −2 oocyte, in C. briggsae it was only apparent in the −1 oocyte, and in C. remanei, AIR-2 was evident at the midbivalent region in −3 to −1 oocytes (Figure 2A). C. brenneri AIR-2 is 69% identical to Cel-AIR-2 (vs. C. briggsae 82% and C. remanei 80%) and displays no identity with the region used for antibody production (Figure S1B); consequently, no specific staining was observed with the Cel-AIR-2 antibodies. To probe the bivalent structure of C. brenneri oocytes, we stained oogenic germ lines with antibodies that recognize the phosphorylated form of histone 3 serine 10 (pH3S10), which is phosphorylated by AIR-2 (Hsu et al. 2000). Histones are highly conserved and pH3S10 has previously been shown to also be concentrated on the short arm of the bivalent in C. elegans oocytes (Collette et al. 2011). pH3S10 was exclusively on the short arm of bivalents in C. elegans, concentrated on the bivalent short arms in C. remanei and C. brenneri oocytes and detectable on the short arm of bivalents in C. briggsae oocytes. Additionally, and in contrast to C. elegans, different extents of pH3S10 were detected around the entire bivalent in these other species (Figure 2C), suggesting additional kinases may be phosphorylating H3S10 and/or that AIR-2 is present around the bivalent below detectable levels. LAB-1, a marker for the long arm (de Carvalho et al. 2008), is poorly conserved among the species (40–48% identity with Cel-LAB-1) precluding analysis. Nonetheless, these results suggest that chromosomes are restructured into a short and long arm consistent with formation of a single crossover on each chromosome pair in all species.
Figure 2.
Conservation of bivalent restructuring suggests a single crossover is formed per chromosome pair in Caenorhabditis. (A) Immunolocalization of AIR-2 (cyan) in C. elegans, C. briggsae, and C. remanei female/hermaphrodite at −3, −2, and −1 position of diakinesis (see Figure 1A). C. brenneri diakinesis nuclei were omitted as no specific staining was observed with the Cel-AIR-2 antibody. The various positions (−3, −2, and −1) of diakinesis indicate its position relative to the spermatheca, with the oocyte in the −1 position being the most mature and closest to the spermatheca. Bar, 5 µM. (B) A blow-up of a single −1 diakinesis-stage bivalent for C. elegans, C. briggsae, and C. remanei. Bar, 1 µM. (C) Immunolocalization of pH3S10 (cyan) in C. elegans, C. briggsae, C. remanei, and C. brenneri female/hermaphrodite at −1 position of diakinesis with a blow-up of a single bivalent. (D) LP, mid-diplotene, and −3 to −1 position diakinesis nuclei stained with DAPI (red) and either anti-SYP-2 (cyan) for C. elegans and C. briggsae or anti-SYP-1 (cyan) for C. remanei and C. brenneri. Yellow arrows point to SYP polycomplex. Bar, 5 µM. (E) A blow-up of a single −3 diakinesis-stage bivalent for C. elegans and C. briggsae. Bar, 1 µM.
In C. elegans, central components of the SC are asymmetrically disassembled during late prophase and retained on the short arm of bivalents in the −3 oocyte (de Carvalho et al. 2008). To further examine restructuring of the bivalent, we monitored the disassembly of SC central components in all four species at LP using anti-peptide antibodies generated against C. elegans SYP-1, which also recognizes C. remanei and C. brenneri SYP-1 and C. elegans SYP-2, which also recognizes C. briggsae SYP-2 (Figure S1, C and D) (Larson et al. 2016). As all SYPs are interdependent for SC assembly (Colaiácovo et al. 2003; Smolikov et al. 2007b, 2009), we hypothesized that all would behave the same, even though we were limited to examining a single SYP in each species due to the extent of SYP identity between the species. In C. briggsae, SYP-2 was partially disassembled in diplotene and appeared to be retained at the intersection between some of the homologous chromosome pairs in −3 oocytes (Figure 2, D and E). Note that the chromosome pairs appear very extended at this stage in C. briggsae. In both C. elegans and C. briggsae, no SYP-2 was detected on −2 oocyte chromosomes. In contrast to C. elegans and C. briggsae, we did not observe asymmetric removal of SYP-1 in C. remanei or C. brenneri at the corresponding stages of meiotic prophase. In C. remanei, SYP-1 was largely removed from chromosomes in diplotene and formed a SYP-1 aggregate/polycomplex not associated with chromatin in −3 oocytes (Figure 2D, yellow arrows). In C. brenneri, SYP-1 was partially removed by diplotene and no SYP-1 was observed in the −3 oocyte. These results suggest that while bivalents are restructured, there are differences in SC disassembly among these Caenorhabditis species.
Levels of X-specific RAD-51 foci are strikingly different in C. elegans and C. briggsae vs. C. remanei and C. brenneri male germ cells
During C. elegans meiosis, X chromosomes have several unique properties, including accumulation of repressive chromatin marks, transcriptional silencing, and alteration of the recombination landscape (Reinke et al. 2000; Reuben and Lin 2002; Bean et al. 2004; Rockman and Kruglyak 2009; Jaramillo-Lambert and Engebrecht 2010; Checchi et al. 2014). Although there are different repressive chromatin marks enriched on X chromosomes in these different Caenorhabditis species, in all cases, they are devoid of the active mark histone 3 lysine 4 dimethylation (H3K4me2) (Larson et al. 2016). To specifically monitor DSB repair on X chromosomes, we costained germ lines with antibodies recognizing RAD-51 and H3K4me2. RAD-51 was clearly present on the X chromosome pair during oogenesis in all Caenorhabditis species, ranging from 8 to 25% of total RAD-51 foci at pachytene (Figure 3). If DSBs as marked by RAD-51 were evenly distributed at pachytene between all chromosome pairs, which are relatively equal in size (Hillier et al. 2007), we would expect the same number of RAD-51 foci on the X chromosome pair as on an autosome pair (X RAD-51 foci to autosome RAD-51 ratio of 1). In hermaphrodite/female germ cells, we observed ratios of 1.09 (C. elegans), 1.67 (C. briggsae), 0.43 (C. remanei), and 0.67 (C. brenneri), suggesting that DSBs are not evenly distributed. In particular, significantly fewer RAD-51 foci are on the X compared to the autosomes at pachytene in the female/male species (Figure 3A, Figure S5A, and Figure S6B). These differences could reflect differences in distribution, timing of break formation, and/or kinetics of repair and may be influenced by the heterochromatin-like state of the X as previously suggested for C. elegans (Gao et al. 2015).
Figure 3.
X-specific RAD-51 in Caenorhabditis germ cells. (A and B) C. elegans, C. briggsae, C. remanei, and C. brenneri female/hermaphrodite and male EP to MP nuclei stained with DAPI (blue), anti-H3K4me2 (green), and anti-RAD-51 (red). White dashed circles denote the X chromosome(s) identified by absence of H3K4me2. Pie graphs comparing percentage of total RAD-51 found on the X chromosome(s) for each species. Below the pie graphs is the average number of RAD-51 on the X chromosome(s) with the standard error of mean. A minimum of three germ lines were examined for each species. (C) A rare C. remanei and C. brenneri nuclei with RAD-51 localized to the male X chromosome. (D) C. nigoni and C. tropicalis male pachytene nuclei stained with DAPI (blue), anti-H3K4me2 (green), and RAD-51 (red). White dashed circles denote the X chromosome identified by absence of H3K4me2. Pie graphs comparing percentage of total RAD-51 found on the X chromosome for each species. All bars, 5 µM.
We observed a more extreme situation in male germ cells, with significantly fewer RAD-51 foci on the single X chromosome of males in the female/male species compared to the hermaphrodite/male species (Figure 3, B and C and Figure S5B). Twenty-one percent and 14% of RAD-51 foci were observed on the X chromosome of males at pachytene in C. elegans and C. briggsae, respectively. On the other hand, 0.6 and 1% of RAD-51 foci were observed on the single X in C. remanei and C. brenneri male pachytene germ cells, respectively (Figure 3B). The X:A ratios were 2.38 (C. elegans), 1.67 (C. briggsae), 0.06 (C. remanei), and 0.10 (C. brenneri). These ratios are per chromosome to account for the single X chromosome vs. the two chromosomes per autosome pair and are significantly different between the androdioecious vs. gonochoristic species (Figure S6A). The differences are likely a consequence of both the higher numbers of RAD-51 on the autosomes and the reduced numbers of RAD-51 on the X chromosome. Indeed, the average number of RAD-51 on the X were also significantly lower in the female/male vs. hermaphroditic species (Figure S5 and Figure S6). Further, the X:A ratios and average RAD-51 on the X chromosome(s) of the males vs. the hermaphrodites/females were highly statistically different in the gonochoristic species (Figure S6C). In the female/male C. nigoni, we also observed very few breaks on the X (Figure 3D, 0.43% of RAD-51 on X). In the hermaphroditic/male species C. tropicalis, we saw low but detectable levels of RAD-51 on the X chromosome of males, which was statistically different from both the other hermaphrodite/male species and the female/male species (C. tropicalis vs. C. remanei, C. brenneri, C. nigoini, P < 0.05; C. tropicalis vs. C. elegans, C. briggsae, P < 0.0001, Student’s t-test; Figure 3D). Nonetheless, although overall levels of RAD-51 are higher in gonochoristic species, many fewer RAD-51 foci are detected on the X, suggesting that the X chromosome is not a good substrate for the meiotic DSB machinery in C. remanei and C. brenneri.
X chromosome pseudosynapsis and DNA repair pathway choice
We previously reported that the single X chromosome of C. elegans male germ cells undergoes transient pseudosynapsis early in prophase and has increased flexibility of DSB repair pathway choice compared to autosomes (Checchi et al. 2014). We proposed that these alterations allow for accommodation of hemizygosity. Our analysis of RAD-51 loading and disassembly on X chromosomes above suggested that there could be alternative strategies for dealing with hemizygosity in these related species. To determine whether the X chromosome of males in these other species also underwent pseudosynapsis, we stained male germ lines with antibodies directed against SC central region components, SYP-1 or SYP-2 (MacQueen et al. 2002; Colaiácovo et al. 2003), which we previously reported recognize the corresponding SYPs in these species but is not present on the X chromosome in pachytene nuclei (Larson et al. 2016).
In C. elegans, transient SYP loading on the X chromosome of males occurs in the TZ where chromosomes are not well separated and acquisition of X-specific chromatin marks is being established. Consequently, the X chromosome can only be unambiguously identified in a subset of TZ nuclei by absence of H3K4me2. Consistent with our previous analysis of SYP-1 (Checchi et al. 2014), 9.4% of TZ nuclei where the X could be identified contained SYP-2 on the X chromosome of C. elegans males (Figure 4). In contrast to C. elegans, we detected no SYP-1 on the X in C. remanei and C. brenneri in those TZ nuclei where the X could be identified (Figure 4, of >100 nuclei scored). On the other hand, while the majority of C. briggsae male TZ germ cells contained no SYP-2 staining on the X chromosome, 3.7% of TZ nuclei where the X could be identified contained SYP-2. These findings suggest that the X chromosome of C. elegans and C. briggsae male germ cells undergoes transient pseudosynapsis and loads RAD-51 at meiotic DSBs, while neither pseudosynapsis nor RAD-51 foci are observed on the X chromosomes of C. remanei and C. brenneri male germ cells.
Figure 4.
Transient pseudosynapsis of the X chromosome in C. elegans and C. briggsae male germ cells . TZ nuclei stained with DAPI (blue), H3K4me2 (green), and either anti-SYP-2 (red) for C. elegans and C. briggsae or anti-SYP-1 (red) for C. remanei and C. brenneri. White dashed circles denote the single X chromosome of males based on the absence of H3K4me2 staining. Number of TZ nuclei examined from a minimum of six germ lines. C. elegans, 149; C. briggsae, 107; C. remanei, 116; and C. brenneri, 125. Bar, 5 µM.
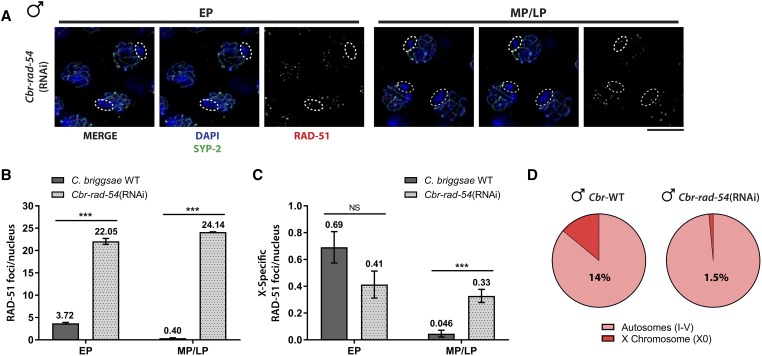
We next addressed whether repair of X-specific breaks can proceed when HR is blocked in C. briggsae male germ cells as in C. elegans. To that end, we depleted Cbr-RAD-54 using the C. briggsae strain susceptible to RNAi by feeding (Nuez and Felix 2012). RAD-54 is essential for RAD-51-mediated strand exchange during HR and is required for RAD-51 disassembly (Solinger et al. 2002; Mets and Meyer 2009); consequently, inactivation of RAD-54 results in high levels of RAD-51 because DSBs cannot be repaired by HR, remain loaded with RAD-51, and signal for additional break formation because a crossover cannot be formed (Rosu et al. 2013; Stamper et al. 2013). To test the efficiency of RNAi, we monitored progeny viability in worms propagated on RNAi medium for two generations and observed greatly increased inviability in the presence of Cbr-RAD-54 dsRNA [Cbr-WT (L4440 control): 2.7 ± 0.8% vs. Cbr-rad-54(RNAi): 95.3 ± 1.7% inviable progeny, P < 0.0001], consistent with efficient knockdown of Cbr-RAD-54.
As the RNAi feeding strain has two genomic regions that lack H3K4me2, the X chromosome and the integrated Cel-sid-2 array on chromosome V, we monitored X-specific RAD-51 foci by costaining with SYP-2, which is not on the X in pachytene germ cells (Larson et al. 2016). Analysis of RAD-51 foci in Cbr-rad-54(RNAi) worms revealed high levels of RAD-51 foci genomewide (Figure 5, A and B); however, there was only a modest increase of X-specific RAD-51 foci in mid–late pachytene (Figure 5, A and C). While this data suggests that Cbr-RAD-54 contributes to repair of X-specific breaks, it is likely that RAD-51 was disassembled on at least a subset of X DSBs in the absence of Cbr-RAD-54, as a significantly smaller percentage of the total RAD-51 foci localized to the X during pachytene [Figure 5D, 1.5% in Cbr-rad-54(RNAi) vs. 14% in wild-type males, P < 0.001, Pearson’s χ2 test]. Thus similar to C. elegans, meiotic DSBs on the X chromosome can be repaired in the absence of RAD-54 in C. briggsae male germ cells.
Figure 5.
RAD-51 disassembly on the C. briggsae male X chromosome in the absence of RAD-54. (A) EP and MP/LP nuclei stained with DAPI (blue), anti-SYP-2 (green), and anti-RAD-51 (red). White dashed circles denote the X chromosome identified by absence of SYP-2. Bar, 5 µM. (B and C) Comparison of total genomewide RAD-51 and RAD-51 on the X chromosome from C. briggsae wild-type and C. briggsae worms treated with cbr-rad-54(RNAi). Error bars = SEM. NS, nonsignificant; *** P < 0.0001, Student’s t-test. (D) Pie graph with the percentage of total RAD-51 found on the X chromosome during pachytene in C. briggsae wild-type and cbr-rad-54(RNAi) males.
The X chromosome of C. remanei and C. brenneri males is not a substrate of the DSB formation machinery
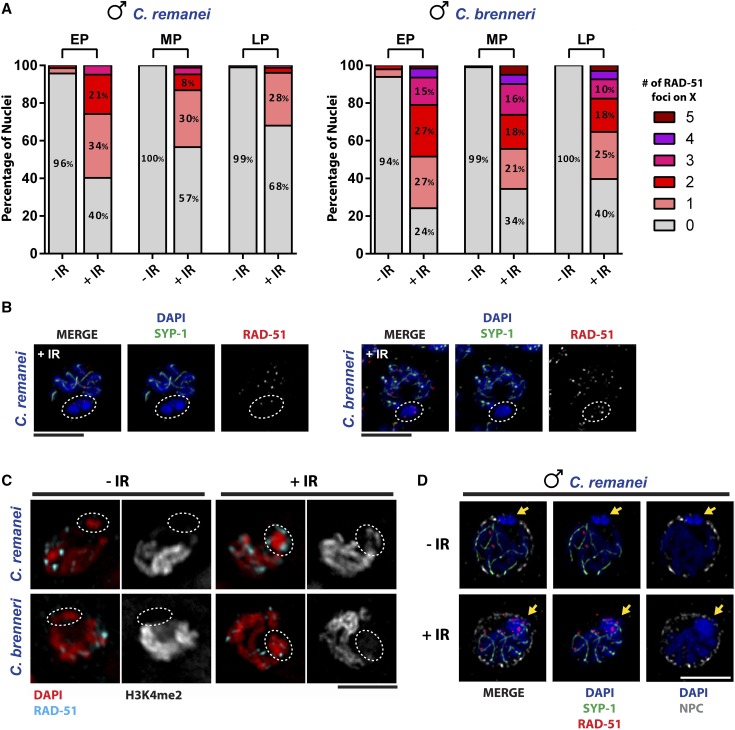
In contrast to C. elegans and C. briggsae, very few RAD-51 foci were observed on the X chromosome of C. remanei and C. brenneri male germ cells (Figure 3, <1%). Because the X chromosome is enriched for heterochromatin marks and highly compacted, antibody accessibility could be an issue, even though antibodies directed against heterochromatin marks are readily detected on the X in male germ cells in these species (Larson et al. 2016). Alternatively, the X chromosome may not be subject to SPO-11-dependent breaks. To distinguish between these possibilities, we treated worms with γ-IR to induce DNA breaks; in the C. elegans germ line, IR-induced breaks are loaded with RAD-51 (Polanowska et al. 2006; Hayashi et al. 2007). We exposed worms to 10 Gy of IR, which in C. elegans results in ∼3.9 breaks/chromosome pair (Yokoo et al. 2012), thus we would predict fewer than two breaks on the single X chromosome of males. We dissected germ lines 30 min following IR exposure and stained extruded germ lines with RAD-51 antibodies. We saw a significant increase in RAD-51 foci on the X at all stages of meiotic prophase in both C. remanei and C. brenneri (Figure 6, A and B, −IR vs. +IR, P < 0.001, t-test). In both species, there were more RAD-51 foci on the X in EP compared to LP (average RAD-51 foci on X: C. remanei, EP: 0.9 ± 0.11; MP: 0.64 ± 0.10; LP: 0.37 ± 0.07; C. brenneri, EP: 1.53 ± 0.16; MP: 1.51 ± 0.19; LP: 1.24 ± 0.16). The change in number of RAD-51 foci throughout pachytene may reflect the engagement of different repair pathways late in prophase, as has been observed in C. elegans (Hayashi et al. 2007; Smolikov et al. 2007a). Additionally, the difference in the number of breaks between C. remanei and C. brenneri may be a consequence of differences in the kinetics of repair of IR-induced breaks. Thus, these results indicate that breaks loaded with RAD-51 on the X chromosome of C. remanei and C. brenneri can be detected by antibody staining and suggest that meiotic breaks are not induced on the X. Alternatively, it is possible that meiotic DSBs are induced but are not resected to load RAD-51, which is unlikely, given that RAD-51 is loaded on IR-induced breaks (Figure 6).
Figure 6.
IR-induced breaks load RAD-51 on the X chromosome of C. remanei and C. brenneri male germ cells. (A) Graph showing the percentage of nuclei with 0–5 RAD-51 foci on the X chromosome of C. remanei and C. brenneri male germ cells before and after IR at EP, MP, and LP. Worms were treated with 10 Gy of IR and allowed to recover for 30 min. A minimum of 60 nuclei were examined for each species and condition. (B) Pachytene nuclei stained with DAPI (blue), anti-SYP-1 (green), and anti-RAD-51 (red) in C. remanei and C. brenneri males treated with 10 Gy of IR and allowed to recover for 30 min. White dashed circles denote the single X chromosome of males based on the absence of SYP-1 staining. (C) Pachytene nuclei stained with DAPI (red), anti-RAD-51 (cyan), and H3K4me2 (gray) in C. remanei and C. brenneri male germ cells before and after IR. White dashed circles denote the single X chromosome of males based on the absence or partial absence of H3K4me2 staining. (D) Pachytene nuclei stained with DAPI (blue), anti-RAD-51 (red), anti-SYP-1 (green), and NPC (gray) in C. remanei males before and after IR. Image is a single slice through the middle of the nucleus. Yellow arrows mark the single X chromosome of male germ cells based on the absence of SYP staining. All bars, 5 µM.
We also examined RAD-51 foci on the X chromosome over time following exposure to 10 Gy of IR in C. remanei male germ cells (Figure S7). Consistent with the analysis above, RAD-51 was readily detected on the X chromosome after 30 min, levels increased slightly at 2 hr following IR, and then slowly decreased at 4 and 8 hr following IR treatment. These results suggest that it takes significant time to process IR-induced breaks.
In addition to being highly compacted, the X chromosome tends to reside in a separate domain from the bulk of the autosomes and we noted that both the compaction state and relationship to the autosomes appeared altered upon IR treatment (Figure 6C). Consistent with a change in compaction, X chromosomes loaded with RAD-51 following IR contained low levels of the activating mark, H3K4me2 (Figure 6C). To explore the spatial configuration of the X in more detail, we costained extruded germ lines isolated from worms before and after IR treatment with an antibody that recognizes the nuclear pore complex (NPC), SYP-1 to identify the X, and RAD-51 (Figure 6D). In the absence of IR, the X chromosome was very compact and was in close proximity to the nuclear envelope in such a manner as to exclude NPCs (Figure 6D, −IR). Upon IR treatment, the X chromatin appeared much more diffuse and was in the same domain as the autosomes. Additionally, the X appeared to have moved inward such that NPCs were no longer excluded from the region adjacent to the X chromosome. Thus, in response to IR, the X chromosome is altered both in terms of chromatin state and nuclear position, presumably to accommodate break repair.
Discussion
Here we show that while many aspects of meiosis are conserved, there are a number of differences in the meiotic process among Caenorhabditis species. Strikingly, we discovered that these different species have adopted distinct strategies to accommodate the hemizygous X chromosome during male meiosis. These results suggest that in addition to sex chromosomes having evolved independently in multiple lineages, molecular pathways promoting transmission of hemizygous sex chromosomes through meiosis have diverged substantially even within the same lineage.
DSB levels vary considerably between Caenorhabditis species
We previously showed that the overall meiotic program is similar between Caenorhabditis species (Larson et al. 2016). Using RAD-51 foci as an indirect readout of DSB formation and repair, we show here that there appears to be substantially more meiotic DSBs in C. remanei and C. brenneri, compared to C. elegans and C. briggsae. Differences in repair kinetics are also likely to contribute to the numbers of RAD-51 observed. The difference in RAD-51 foci between female/male vs. hermaphrodite/male species is not merely a consequence of the decrease in genome size attributed to hermaphroditism (Fierst et al. 2015), as we observe two to three times the number of RAD-51 foci in C. remanei and C. brenneri, which have only ∼30% larger genomes compared to C. elegans and C. briggsae. Environmental differences, particularly temperature, have also been shown to influence recombination (Bomblies et al. 2015), suggesting that the differences in DSBs could be a consequence of these species’ ecology. However, the Caenorhabditis species examined here have been shown to cohabitate and are relatively cosmopolitan in their distribution, except C. brenneri, which is restricted to tropical zones (Kiontke et al. 2011). Thus, there is no simple correlation with either genome size or habitat that explains the difference in level of DSBs. Could these differences be a consequence of the different reproductive lifestyles of these nematodes? Perhaps hermaphroditic selfing selects against high numbers of DSBs, as DSBs are unlikely to generate genetic variation and only a single crossover is formed per chromosome pair. Consistent with this, analysis of gonochoristic C. nigoni, a sister species of C. briggsae that can produce fertile interspecies offspring with C. briggsae (Woodruff et al. 2010; Kiontke et al. 2011), also shows high levels of RAD-51 foci, while the other hermaphrodite/male species within the elegans group, C. tropicalis, shows low levels (Figure 1H). Thus, among the species examined here, reproductive mode appears to correlate with meiotic DSB levels.
DSBs are not evenly distributed along the genome, but tend to be enriched at hotspots. Recent genomic analyses across Saccharomyces yeast species revealed a remarkable level of conservation of both the strength and position of DSB hotspots (Lam and Keeney 2015). In this taxa, DSBs are targeted to promoters suggesting that the conservation of hotspots reflects chromosomal feature (i.e., promoters) that are under evolutionary constraint. A similar conservation of hotspots has also been reported in birds (Singhal et al. 2015). These results contrast with the remarkable level of divergence of DSB hotspots in mammals, which rely on the zinc finger histone H3K4 methylase, PRDM9 (reviewed in de Massy 2013). As in yeast and birds, Caenorhabditis does not have a PRDM9 ortholog; however, recombination mapping and preliminary RAD-51 chromatin immunoprecipitation analyses in C. elegans suggest that there are no hotspots per se, although RAD-51 is enriched at actively transcribing genes (Rockman and Kruglyak 2009; Kaur and Rockman 2014; Yu et al. 2016). The absence of hotspots may allow for flexibility in the levels, and perhaps position, of DSBs in these different species. Ultimately, DSBs will need to be mapped at the molecular level to determine what chromosomal features facilitate DSB formation in Caenorhabditis, and whether this is altered between species. There is precedence for differences in recombination between species in the same genus as illustrated in Drosophila where D. melanogaster males do not exhibit any meiotic recombination, while some populations of D. ananassae do (Goni et al. 2016). Further, recombination can vary widely between the sexes, and even within individuals of the same species (Fledel-Alon et al. 2011).
Single crossover per chromosome pair as an adaptation to holocentry
In all species examined, many more DSBs are induced than will be processed into crossovers (Lake and Hawley 2016). In some species, this reflects the role of DSB repair in the process of chromosome pairing (reviewed in Zickler and Kleckner 2015). However, in C. elegans and Drosophila, where chromosome pairing can occur in the absence of meiotic recombination (Dernburg et al. 1998; McKim and Hayashi-Hagihara 1998), there are still more DSBs than crossovers (Colaiácovo et al. 2003; Mehrotra and McKim 2006). The relationship between DSBs and crossovers is complex, as enough DSBs must be induced to ensure that every chromosome receives a crossover for disjunction. Conversely, excess crossovers are deleterious and are inhibited by the process of crossover interference (reviewed in Zickler and Kleckner 2016). C. elegans represents an extreme case of crossover interference as on average only a single crossover is formed per chromosome pair (Meneely et al. 2002; Hillers and Villeneuve 2003). We provide evidence that strong crossover interference is likely a conserved feature of Caenorhabditis and is perhaps even more potent in those species with more DSBs. We propose that limiting crossovers to one per chromosome pair is an adaptation to holocentry in Caenorhabditis. Beautiful work in C. elegans has shown that the single crossover per chromosome pair in meiosis is essential for the restructuring of the bivalent into a long and short arm (Nabeshima et al. 2005). Restructured bivalents allow for regulated cohesin release on the short arms, and in combination with kinetochores forming a cup-like structure around the long arm ends, ensure proper chromosome segregation during meiosis (de Carvalho et al. 2008; Dumont et al. 2010). Although restructuring of the bivalent is conserved among Caenorhabditis (Figure 2), the details are different between the species. In particular, disassembly of SC central region components has a distinct pattern in each species. Whether this is a consequence of differences in the number of diplotene/diakinesis nuclei (Larson et al., 2016), or an inherent difference in how the SC is disassembled, is not known.
Consistent with formation of a single crossover per chromosome pair, in C. elegans each chromosome has a genetic length of ∼50 cM. Genomic analyses and recombinant inbred lines have been used to generate a genetic map for C. briggsae, which are also consistent with formation of a single crossover per chromosome (Hillier et al. 2007; Ross et al. 2011). Additionally, several chromosomal features, such as less recombination in the center compared to the arms, suggest that the crossover landscape is similar between these species. Much more limited analysis has been performed on C. remanei and C. brenneri, but it is likely that they are also limited to a single crossover per chromosome arm to ensure disjunction, consistent with our analysis of bivalent restructuring.
Different strategies to accommodate sex chromosome hemizygosity
Remarkably, we show that species within the Caenorhabditis genus use very different strategies to accommodate sex chromosome hemizygosity, even though the emergence of sex chromosomes likely predated the Caenorhabditis lineage. The single X chromosome of males in both C. elegans and C. briggsae germ cells undergoes a transient period of pseudosynapsis contemporaneous with DSB formation (Checchi et al. 2014) (Figure 4). Further, in both species, break repair can precede in the absence of HR (Checchi et al. 2014) (Figure 5). Thus, in C. elegans and C. briggsae we propose that hemizygosity is accommodated by the X sister chromatids masquerading as an autosome pair early in meiosis and as meiosis proceeds, the constraints on DSB repair are relaxed. In C. tropicalis, the other independently derived hermaphrodite/male species, we observed significantly fewer breaks on the X than C. elegans or C. briggsae, but more than in the female/male species. Perhaps C. tropicalis represents an intermediate state between C. elegans and C. briggsae vs. the female/male species. The predicted phylogeny of the elegans group suggests that C. tropicalis diverged from its gonochoristic ancestor longer ago than C. briggsae (Kiontke et al. 2011). Thus it is equally likely that C. tropicalis uses a different strategy to accommodate sex chromosome hemizygosity than either the other hermaphroditic species or the three gonochoristic species we examined here.
The lack of DSBs, as marked by RAD-51, on the C. remanei and C. brenneri (and C. nigoni) X chromosome of males suggests that SPO-11 can be prevented from breaking a chromosome, which would be advantageous when a homologous chromosome is not present as a template for repair. While we show that SYP-1 is not loaded onto these chromosomes, it remains to be determined whether axial components are loaded onto the X as they are in C. elegans (Jaramillo-Lambert and Engebrecht 2010), and we presume in C. briggsae. Of particular interest would be to assess whether the HORMA domain protein, HTP-3, which is essential for DSB formation (Goodyer et al. 2008), is present on the X chromosome of C. remanei and C. brenneri. If HTP-3 is not loaded on the X, this would provide an explanation for why SPO-11 does not induce breaks. However, as HTP-3 is loaded very early during meiosis prior to chromosome pairing, it would be surprising if HTP-3 was not loaded on the X. Unfortunately, axial components are not well conserved and therefore we were unable to examine HTP-3 in these other species.
One feature of sex chromosomes that is likely to influence breakage and repair is their repressive chromatin nature. It is well documented that repair kinetics and repair pathway choice are distinct in euchromatin vs. heterochromatin (Xu and Price 2011). Further, previous analysis indicated that the X chromosome pair in C. elegans hermaphroditic germ cells had fewer DSBs than the autosomes as a consequence of the unique chromatin landscape of the X (Gao et al. 2015). However, although enriched for repressive chromatin marks, the X is not a classic heterochromatin domain with repetitive sequences. Further, it is unlikely that a single repressive mark dictates either the extent of breakage or mode of repair, as the X chromosome of C. elegans (RAD-51+) and C. remanei (RAD-51−) is enriched for H3K9me2 while the X chromosome of C. briggsae (RAD-51+) and C. brenneri (RAD-51−) is enriched for H3K9me3 (Larson et al. 2016). Consistent with this, inactivation of MET-2, a SETBD1 histone methyltransferase responsible for acquisition of these repressive chromatin marks, does not alter meiotic DSB levels in C. elegans (Checchi and Engebrecht 2011), although MET-2 may influence DSB distribution (Yu et al. 2016).
Recent work in both Drosophila and mammalian tissue culture has shown that DSBs within heterochromatin domains move outside of the heterochromatic region for repair by HR (Chiolo et al. 2011; Ryu et al. 2015; Tsouroula et al. 2016). In both of these systems, HR occurs at the nuclear periphery. We provide evidence that the X chromosome of C. remanei and C. brenneri male germ cells is sequestered at the nuclear periphery where we propose SPO-11 and associated proteins do not have access. Our analysis of IR-induced breaks in C. remanei and C. brenneri suggest that the X chromosome also undergoes movement in response to DNA breaks (Figure 6); however, in contrast to what is observed in Drosophila and mammals, the X chromosome appears to move away from the nuclear periphery. An important distinction is that we are examining meiotic cells where recombination is actively engaged vs. somatic cells, where DSBs are rarely purposefully inflicted on the genome. Further, chromosomes have distinct configurations in meiosis compared to somatic cells, perhaps altering the relationship to the nuclear periphery. Nonetheless, in all of these examples, movement is accompanied by a change in chromatin state. Thus, although there are parallels between the behavior of the X of C. remanei and C. brenneri males and these classic heterochromatin domains, there are also differences. Whether this reflects genome size and complexity or unique aspects of meiotic cells generally, or sex chromosomes specifically, is not currently known.
Acknowledgments
We thank Jill Shumacher (University of Texas), Sarit Smolikove (University of Iowa), and Anne Villeneuve (Stanford University) for AIR-2, SYP-1/-2, and RAD-51 antibodies, respectively; Katherine Lawrence for discussions; Qianyan Li for help with imaging; and Ryan Jensen for construction of RNAi feeding clones. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40-OD010440). This work was supported by NIH grant GM-103860 and the Agricultural Experimental Station California-Davis grant *MCB-7237-H (to J.E.).
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.194308/-/DC1.
Communicating editor: M. P. Colaiácovo
Literature Cited
- Albertson D. G., Rose A. M., Villeneuve A. M., 1997. Chromosome organization, mitosis, and meiosis, in C. elegans II, edited by Riddle D. L., Blumenthal T., Meyer B. J., Priess J. R., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- Alpi A., Pasierbek P., Gartner A., Loidl J., 2003. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112: 6–16. [DOI] [PubMed] [Google Scholar]
- Bean C. J., Schaner C. E., Kelly W. G., 2004. Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nat. Genet. 36: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Park D., Xu L., Kleckner N., 1992. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69: 439–456. [DOI] [PubMed] [Google Scholar]
- Bomblies K., Higgins J. D., Yant L., 2015. Meiosis evolves: adaptation to external and internal environments. New Phytol. 208: 306–323. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Bishop D. K., 2015. DNA strand exchange and RecA homologs in meiosis. Cold Spring Harb. Perspect. Biol. 7: a016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchi P. M., Engebrecht J., 2011. Caenorhabditis elegans histone methyltransferase MET-2 shields the male X chromosome from checkpoint machinery and mediates meiotic sex chromosome inactivation. PLoS Genet. 7: e1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchi P. M., Lawrence K. S., Van M. V., Larson B. J., Engebrecht J., 2014. Pseudosynapsis and decreased stringency of meiotic repair pathway choice on the hemizygous sex chromosome of Caenorhabditis elegans males. Genetics 197: 543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolo I., Minoda A., Colmenares S. U., Polyzos A., Costes S. V., et al. , 2011. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan A., 2005. Nematode genome evolution. WormBook 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaiácovo M. P., MacQueen A. J., Martinez-Perez E., McDonald K., Adamo A., et al. , 2003. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell 5: 463–474. [DOI] [PubMed] [Google Scholar]
- Cole F., Keeney S., Jasin M., 2010. Evolutionary conservation of meiotic DSB proteins: more than just Spo11. Genes Dev. 24: 1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette K. S., Petty E. L., Golenberg N., Bembenek J. N., Csankovszki G., 2011. Different roles for Aurora B in condensin targeting during mitosis and meiosis. J. Cell Sci. 124: 3684–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho C. E., Zaaijer S., Smolikov S., Gu Y., Schumacher J. M., et al. , 2008. LAB-1 antagonizes the Aurora B kinase in C. elegans. Genes Dev. 22: 2869–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Massy B., 2013. Initiation of meiotic recombination: How and where? Conservation and specificities among eukaryotes. Annu. Rev. Genet. 47: 563–599. [DOI] [PubMed] [Google Scholar]
- Dernburg A. F., McDonald K., Moulder G., Barstead R., Dresser M., et al. , 1998. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94: 387–398. [DOI] [PubMed] [Google Scholar]
- Dumont J., Oegema K., Desai A., 2010. A kinetochore-independent mechanism drives anaphase chromosome separation during acentrosomal meiosis. Nat. Cell Biol. 12: 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierst J. L., Willis J. H., Thomas C. G., Wang W., Reynolds R. M., et al. , 2015. Reproductive mode and the evolution of genome size and structure in Caenorhabditis nematodes. PLoS Genet. 11: e1005323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledel-Alon A., Leffler E. M., Guan Y., Stephens M., Coop G., et al. , 2011. Variation in human recombination rates and its genetic determinants. PLoS One 6: e20321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Kim H. M., Elia A. E., Elledge S. J., Colaiácovo M. P., 2015. NatB domain-containing CRA-1 antagonizes hydrolase ACER-1 linking acetyl-CoA metabolism to the initiation of recombination during C. elegans meiosis. PLoS Genet. 11: e1005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V., Phelps S. E., Gray S., Neale M. J., 2011. Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature 479: 241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni B., Matsuda M., Tobari Y. N., 2016. Male recombination in Brazilian populations of Drosophila ananassae. Genome 59: 493–500. [DOI] [PubMed] [Google Scholar]
- Goodyer W., Kaitna S., Couteau F., Ward J. D., Boulton S. J., et al. , 2008. HTP-3 links DSB formation with homolog pairing and crossing over during C. elegans meiosis. Dev. Cell 14: 263–274. [DOI] [PubMed] [Google Scholar]
- Hammarlund M., Davis M. W., Nguyen H., Dayton D., Jorgensen E. M., 2005. Heterozygous insertions alter crossover distribution but allow crossover interference in Caenorhabditis elegans. Genetics 171: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Chin G. M., Villeneuve A. M., 2007. C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression. PLoS Genet. 3: e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillers K. J., Villeneuve A. M., 2003. Chromosome-wide control of meiotic crossing over in C. elegans. Curr. Biol. 13: 1641–1647. [DOI] [PubMed] [Google Scholar]
- Hillier L. W., Miller R. D., Baird S. E., Chinwalla A., Fulton L. A., et al. , 2007. Comparison of C. elegans and C. briggsae genome sequences reveals extensive conservation of chromosome organization and synteny. PLoS Biol. 5: e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J. Y., Sun Z. W., Li X., Reuben M., Tatchell K., et al. , 2000. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102: 279–291. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Lambert A., Ellefson M., Villeneuve A. M., Engebrecht J., 2007. Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev. Biol. 308: 206–221. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Lambert A., Engebrecht J., 2010. A single unpaired and transcriptionally silenced X chromosome locally precludes checkpoint signaling in the Caenorhabditis elegans germ line. Genetics 184: 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna S., Pasierbek P., Jantsch M., Loidl J., Glotzer M., 2002. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr. Biol. 12: 798–812. [DOI] [PubMed] [Google Scholar]
- Kauppi L., Barchi M., Baudat F., Romanienko P. J., Keeney S., et al. , 2011. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science 331: 916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T., Rockman M. V., 2014. Crossover heterogeneity in the absence of hotspots in Caenorhabditis elegans. Genetics 196: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S., Giroux C. N., Kleckner N., 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375–384. [DOI] [PubMed] [Google Scholar]
- Kelly W. G., Schaner C. E., Dernburg A. F., Lee M. H., Kim S. K., et al. , 2002. X-chromosome silencing in the germline of C. elegans. Development 129: 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiontke K., Fitch D. H., 2005. The phylogenetic relationships of Caenorhabditis and other rhabditids. WormBook 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiontke K. C., Felix M. A., Ailion M., Rockman M. V., Braendle C., et al. , 2011. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol. Biol. 11: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake C. M., Hawley R. S., 2016. Becoming a crossover-competent DSB. Semin. Cell Dev. Biol. 54: 117–125. [DOI] [PubMed] [Google Scholar]
- Lam I., Keeney S., 2015. Nonparadoxical evolutionary stability of the recombination initiation landscape in yeast. Science 350: 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson B. J., Van M. V., Nakayama T., Engebrecht J., 2016. Plasticity in the meiotic epigenetic landscape of sex chromosomes in Caenorhabditis species. Genetics 203: 1641–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens B. B., Johnson N. M., Tijsterman M., 2013. COM-1 promotes homologous recombination during Caenorhabditis elegans meiosis by antagonizing Ku-mediated non-homologous end joining. PLoS Genet. 9: e1003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui D. Y., Colaiácovo M. P., 2013. Meiotic development in Caenorhabditis elegans. Adv. Exp. Med. Biol. 757: 133–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen A. J., Colaiácovo M. P., McDonald K., Villeneuve A. M., 2002. Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev. 16: 2428–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee B. D., 1996. The license to pair: identification of meiotic pairing sites in Drosophila. Chromosoma 105: 135–141. [DOI] [PubMed] [Google Scholar]
- McKim K. S., Hayashi-Hagihara A., 1998. mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev. 12: 2932–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra S., McKim K. S., 2006. Temporal analysis of meiotic DNA double-strand break formation and repair in Drosophila females. PLoS Genet. 2: e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneely P. M., Farago A. F., Kauffman T. M., 2002. Crossover distribution and high interference for both the X chromosome and an autosome during oogenesis and spermatogenesis in Caenorhabditis elegans. Genetics 162: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets D. G., Meyer B. J., 2009. Condensins regulate meiotic DNA break distribution, thus crossover frequency, by controlling chromosome structure. Cell 139: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M. A., Cohen P. E., 2005. Not all germ cells are created equal: aspects of sexual dimorphism in mammalian meiosis. Reproduction 130: 761–781. [DOI] [PubMed] [Google Scholar]
- Nabeshima K., Villeneuve A. M., Colaiácovo M. P., 2005. Crossing over is coupled to late meiotic prophase bivalent differentiation through asymmetric disassembly of the SC. J. Cell Biol. 168: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuez I., Felix M. A., 2012. Evolution of susceptibility to ingested double-stranded RNAs in Caenorhabditis nematodes. PLoS One 7: e29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkner A., Portik-Dobos Z., Tang L., Schnabel R., Novatchkova M., et al. , 2007. A conserved function for a Caenorhabditis elegans Com1/Sae2/CtIP protein homolog in meiotic recombination. EMBO J. 26: 5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanowska J., Martin J. S., Garcia-Muse T., Petalcorin M. I., Boulton S. J., 2006. A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites. EMBO J. 25: 2178–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V., Smith H. E., Nance J., Wang J., Van Doren C., et al. , 2000. A global profile of germline gene expression in C. elegans. Mol. Cell 6: 605–616. [DOI] [PubMed] [Google Scholar]
- Reuben M., Lin R., 2002. Germline X chromosomes exhibit contrasting patterns of histone H3 methylation in Caenorhabditis elegans. Dev. Biol. 245: 71–82. [DOI] [PubMed] [Google Scholar]
- Rockman M. V., Kruglyak L., 2009. Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet. 5: e1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers E., Bishop J. D., Waddle J. A., Schumacher J. M., Lin R., 2002. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J. Cell Biol. 157: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. A., Koboldt D. C., Staisch J. E., Chamberlin H. M., Gupta B. P., et al. , 2011. Caenorhabditis briggsae recombinant inbred line genotypes reveal inter-strain incompatibility and the evolution of recombination. PLoS Genet. 7: e1002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosu S., Libuda D. E., Villeneuve A. M., 2011. Robust crossover assurance and regulated interhomolog access maintain meiotic crossover number. Science 334: 1286–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosu S., Zawadzki K. A., Stamper E. L., Libuda D. E., Reese A. L., et al. , 2013. The C. elegans DSB-2 protein reveals a regulatory network that controls competence for meiotic DSB formation and promotes crossover assurance. PLoS Genet. 9: e1003674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu T., Spatola B., Delabaere L., Bowlin K., Hopp H., et al. , 2015. Heterochromatic breaks move to the nuclear periphery to continue recombinational repair. Nat. Cell Biol. 17: 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakes D. C., Wu J. C., Sadler P. L., Laprade K., Moore L. L., et al. , 2009. Spermatogenesis-specific features of the meiotic program in Caenorhabditis elegans. PLoS Genet. 5: e1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M., Oh S. D., Hunter N., Shinohara A., 2008. Crossover assurance and crossover interference are distinctly regulated by the ZMM proteins during yeast meiosis. Nat. Genet. 40: 299–309. [DOI] [PubMed] [Google Scholar]
- Singhal S., Leffler E. M., Sannareddy K., Turner I., Venn O., et al. , 2015. Stable recombination hotspots in birds. Science 350: 928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolikov S., Eizinger A., Hurlburt A., Rogers E., Villeneuve A. M., et al. , 2007a Synapsis-defective mutants reveal a correlation between chromosome conformation and the mode of double-strand break repair during Caenorhabditis elegans meiosis. Genetics 176: 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolikov S., Eizinger A., Schild-Prufert K., Hurlburt A., McDonald K., et al. , 2007b SYP-3 restricts synaptonemal complex assembly to bridge paired chromosome axes during meiosis in Caenorhabditis elegans. Genetics 176: 2015–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolikov S., Schild-Prufert K., Colaiácovo M. P., 2009. A yeast two-hybrid screen for SYP-3 interactors identifies SYP-4, a component required for synaptonemal complex assembly and chiasma formation in Caenorhabditis elegans meiosis. PLoS Genet. 5: e1000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger J. A., Kiianitsa K., Heyer W. D., 2002. Rad54, a Swi2/Snf2-like recombinational repair protein, disassembles Rad51:dsDNA filaments. Mol. Cell 10: 1175–1188. [DOI] [PubMed] [Google Scholar]
- Stamper E. L., Rodenbusch S. E., Rosu S., Ahringer J., Villeneuve A. M., et al. , 2013. Identification of DSB-1, a protein required for initiation of meiotic recombination in Caenorhabditis elegans, illuminates a crossover assurance checkpoint. PLoS Genet. 9: e1003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L., Court D. L., Fire A., 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. [DOI] [PubMed] [Google Scholar]
- Tsouroula K., Furst A., Rogier M., Heyer V., Maglott-Roth A., et al. , 2016. Temporal and spatial uncoupling of DNA double strand break repair pathways within mammalian heterochromatin. Mol. Cell 21; 293–305. [DOI] [PubMed] [Google Scholar]
- Woodruff G. C., Eke O., Baird S. E., Felix M. A., Haag E. S., 2010. Insights into species divergence and the evolution of hermaphroditism from fertile interspecies hybrids of Caenorhabditis nematodes. Genetics 186: 997–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Price B. D., 2011. Chromatin dynamics and the repair of DNA double strand breaks. Cell Cycle 10: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Smolikove S., 2013. Impaired resection of meiotic double-strand breaks channels repair to nonhomologous end joining in Caenorhabditis elegans. Mol. Cell. Biol. 33: 2732–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo R., Zawadzki K. A., Nabeshima K., Drake M., Arur S., et al. , 2012. COSA-1 reveals robust homeostasis and separable licensing and reinforcement steps governing meiotic crossovers. Cell 149: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Kim Y., Dernburg A. F., 2016. Meiotic recombination and the crossover assurance checkpoint in Caenorhabditis elegans. Semin. Cell Dev. Biol. 54: 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedek F., Bures P., 2012. Evidence for centromere drive in the holocentric chromosomes of Caenorhabditis. PLoS One 7: e30496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D., Kleckner N., 2015. Recombination, Pairing, and Synapsis of Homologs during Meiosis. Cold Spring Harb. Perspect. Biol. 7: pii a016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D., Kleckner N., 2016. A few of our favorite things: pairing, the bouquet, crossover interference and evolution of meiosis. Semin. Cell Dev. Biol. 54: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]