Abstract
Recent neuroimaging findings in general social anxiety disorder (gSAD) have extended our understanding of the neural mechanisms of gSAD beyond an amygdala-centric fear-based hyperactivity model to include other brain regions and networks relevant to salient cues. In particular, higher order areas compromising visual networks that process emotional and social information have been implicated. The pulvinar anchors this network and is a key regulatory node that mediates complex sensory inputs and the integration between limbic and frontal brain systems. However, the role of the pulvinar and specifically alteration of its effective connectivity with the rest of the brain has not been examined in the pathophysiology of gSAD, a disorder characterized by aberrant socio-emotional processing. The main aim of this study was to examine the pulvinar network effective connectivity in gSAD. In this study, we recruited 21 individuals with gSAD and 19 demographically matched healthy controls (HC), who performed an emotional face processing task while brain activity was recorded using functional magnetic resonance imaging (fMRI). To examine pulvinar-based network dynamics, Granger causality (GC) based effective connectivity (EC) analysis was applied on fMRI data to compare gSAD and HC. The EC analysis revealed heightened casual influential dynamics between pulvinar in higher order visual and frontal regions in gSAD. In conclusion, these preliminary data suggest a novel network-based cortico-pulvino-cortical neural mechanism in the pathophysiology of gSAD.
Keywords: effective connectivity, fMRI, Granger causality, pulvinar, social anxiety
1. Introduction
Generalized social anxiety disorder (gSAD) is associated with an exaggerated and pervasive fear and avoidance of unfamiliar social interaction and possible scrutiny by others.[1] With a lifetime prevalence of 12.1%, gSAD is one of the most common mental disorders in the US adult population.[2] Without proper treatment, gSAD can have significant adverse effect on the quality of life and social functioning. Better understanding of the underlying pathophysiological mechanisms of gSAD is necessary for proper diagnosis and development of effective treatments.
Functional magnetic resonance imaging (fMRI) has been the main methodological approach for studying the neural bases of gSAD. Growing evidence suggests that brain network dysfunction rather than abnormality in isolated brain region (s) underlies the genesis and maintenance of gSAD, similar to other affective disorders.[3,4] The landmark meta-analysis of functional neuroimaging studies by Etkin and Wager in 2007 suggested hyperactivity in a “fear circuit” that includes amygdala, insula, inferior temporal gyrus, fusiform gyrus, and superior temporal gyrus as the core pathologic network dysfunction in gSAD.[5] A recent meta-analysis of brain imaging studies of gSAD expand on the Etkin and Wager findings by incorporating results of recent resting-state connectivity and more advanced structural imaging studies in gSAD.[3] They proposed a network-based model of gSAD that extends beyond fear-related amygdala hyperactivity and that involves other regions, particularly occipital and parietal hubs in the neuropathophysiology of gSAD that may play a broader role in socio-emotional processing.[3]
From a network perspective, one important question is which area(s) of the brain plays a central role in gSAD neurocircuitry and is anatomically connected to and can functionally mediate interactions amongst spatially distributed brain regions? The thalamus, and more specifically pulvinar, is best positioned to play this role. As the largest thalamus nuclei, the pulvinar has recently received a great deal of attention in system neuroscience because of its newly discovered complex and pivotal role in cortico-cortical neural synchronization and information processing needed for higher level cognitive and emotional functions like perceptual attention and sensory emotional processing.[6–9] Functional and structural abnormalities of pulvinar have also been reported in several psychiatric disorders such as depression,[10,11] specific phobia,[12] and obsessive-compulsive disorder (OCD).[13] In a recent published study, we showed pulvinar network dynamics are altered in major depression and can be modulated therapeutically with pharmacological treatment.[11]
In this study, we aim to investigate if pulvinar network dynamics plays a role in the neural pathophysiology of gSAD. We used a Granger causality (GC) based effective connectivity (EC) analytic approach to capture different aspects of pulvinar network causality dynamics during the processing of salient social signals (e.g., emotional faces) in patients with gSAD and healthy controls. Based on the extent literature, we hypothesized that pulvinar network dynamics in gSAD patients will differ from HC in pulvinar EC measures between pulvinar and frontal, limbic, and visual-sensory areas implicated in processing of socio-emotional information.
2. Methods
2.1. Participants
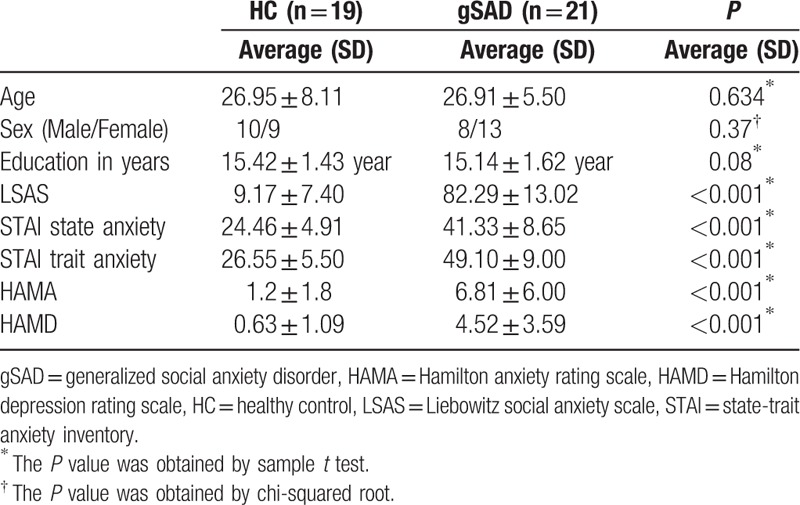
Twenty-one untreated (e.g., unmedicated and not in psychotherapy) gSAD patients and 19 healthy control (HC) volunteers participated in this study. This study was conducted at the University of Chicago (gSAD n = 12; HC n = 14) and at the University of Michigan (gSAD n = 9; HC n = 5). The fMRI data on whole-brain “activation” of the same subjects were previously published.[14] Each subject underwent a screening evaluation involving structured clinical interviews and assessments by trained clinicians and semi-structured medical and psychiatric interviews with the study psychiatrist (KLP). All subjects were characterized with the structured clinical interview for DSM-IV; Liebowitz social anxiety scale (LSAS); Hamilton anxiety rating scale; Spielberger trait-state anxiety inventory; and Hamilton depression rating scale. None of the gSAD subjects had a current/recent depressive episode or alcohol/substance abuse (within 12 months of study entry), or another anxiety disorder that was more clinically salient than generalized social anxiety symptoms. All subjects provided written informed consent, and the study was approved by both local university hospital institutional review boards.
2.2. fMRI task
We used a modified version of the Emotional Face Matching Task (EFMT) to assess pulvinar connectivity dynamics during socio-emotional processing.[15] Briefly, in this task photographs from a validated set of face stimuli[16] were presented in a block-design during which participants view a trio of emotional faces and selected one of two faces (bottom) that expressed the same emotion as the target face (top). The target and congruent probe faces displayed one of three expressions (fearful, angry, or happy), and the other (incongruent) probe face always displayed a neutral/nonemotional expression. Of note, our EC analysis examined brain activity across the entire task (i.e., across all faces blocks [fearful + angry + happy]) for several reasons. First, we took an exploratory approach with the goal to detect gSAD related abnormal pulvinar interaction with any potential brain region and not exclusively the ones that show altered reactivity in specific blocks. Second, the duration of each block was relatively short. By including all tasks blocks, we maximized statistical power of our analysis to detect subtle group differences in abnormal connectivity. Third, there is insufficient data in the literature to propose specific hypothesis that pulvinar network dysfunction would be related to a particular emotional expression (e.g., fearful vs happy faces).
2.3. fMRI data acquisition
This study was conducted on two different 3-Tesla GE Signa System (General Electric, Milwaukee, WI) scanners using the same standard radiofrequency coil: one at the University of Chicago (gSAD n = 12; HC n = 14) and the other at the University of Michigan (gSAD n = 9; HC n = 5). However, all scanning was performed with blood oxygen level-dependent (BOLD)-sensitive whole-brain fMRI using the same GE software (LX 8.3, Neuro Optimizer gradients) and acquired using the exact same T2∗-weighted reverse spiral gradient-recall echo sequence (echo time = 25 ms, repetition time = 2000 ms, 64 × 64 matrix, flip angle = 77° field of view = 24 cm, 3.75 mm2 inplane voxels, 30 contiguous 5-mm axial slices/volume) optimized to minimize susceptibility artifacts in the regions of interest such fronto-limbic nodes. A high-resolution T1 scan was also acquired for anatomic localization for all subjects.
2.4. fMRI data preprocessing
All preprocessing were conducted using statistical parametric mapping software (SPM8, http//www.fil.ion.uvl.ac.uk/spm). The first 4 volumes of the functional images were discarded for obtaining signal equilibrium and allowing participants adaptation to scanning noise. None of the subjects used in this study had more than 2 mm maximum displacement in x, y, or z axis or 2° angular motion during fMRI scanning. Raw EPI images were subsequently realigned, coregistered, normalized, and smoothed with a kernel of 8 mm before statistical analyses.
2.5. Granger causality based effective connectivity analysis
No standardized coordinates for the pulvinar is available, and defining the borders of the pulvinar nucleus solely based on anatomical scans has proven to be difficult.[7] Thus, we used a 5 mm radius sphere a priori centered on the coordinates drawn from Talairach Atlas (right pulvinar: 14–28 8; left pulvinar: −7–25 11) to generate right and left pulvinar seeds for EC analysis.[17] Without any band pass filtering, time series of voxels within each seed region during the entire duration of task performance were averaged as the seed reference time series beside the time series for all other remaining individual voxels in the brain. GC method was applied on extracted time series to examine the causal influence of the right and left pulvinar seeds across the entire brain (pulvinar causal outflow to brain or Pulvinar-to-whole-brain effective connectivity) as well as the causal influence of the rest of the brain on the right and left pulvinar (pulvinar causal inflow from brain or whole-brain-to-pulvinar effective connectivity). We used signed-path coefficients method with a time lag order of 1 (1 TR, 2 s) to determine the probable strength and sign (inhibitory vs excitatory) of causal effect using conventional, validated methods.[18,19] To determine the baseline sign of causal influence (inhibitory vs excitatory) between pulvinar and the significant clusters obtained from group comparison analysis, mean GC coefficient values of all the significant clusters were calculated and subjected to a one-sample t test in healthy control group and all study participants to clarify the direction (positive/excitatory vs negative/inhibitory) of causal effect between the pulvinar and those significant clusters.
2.6. Statistical analysis
Demographic and clinical variables were analyzed for between-group differences using an independent sample t test for continuous variables and chi-squared test for categorical variables. Effective connectivity group differences were also analyzed using independent sample t test. Monte Carlo simulation was applied for multiple comparisons correction using the AlphaSim program.[20] In this study, a corrected whole-brain significance level of P < 0.05 was obtained by using a combination of individual voxel probability threshold P < 0.001 and a minimum cluster size of 13 voxels (or 104 mm3). Two-tailed Pearson correlations were used to explore possible associations between GC based effective connectivity values and symptom severity as indexed with the LSAS.
3. Results
3.1. Demographics and clinical characteristics
Demographic and clinical data of both healthy and gSAD participants are summarized in Table 1. There were no significant differences between the two groups in age, sex, or years of education. As expected, gSAD participants scored significantly higher on general anxiety and social anxiety symptom severity in terms of Hamilton Anxiety, LSAS, Spielberger trait, and state anxiety scores.
Table 1.
Demographic and clinical characteristics of patients and control subjects.
3.2. Alterations in pulvinar effective connectivity in social anxiety disorder
Pulvinar-to-whole-brain EC analyses showed the gSAD group had a significantly higher causal influence of the left pulvinar on the left superior frontal gyrus and right middle occipital gyrus and significantly lower positive causal influence of the right pulvinar on the left middle occipital gyrus, compared with the HC group (Table 2, Fig. 1).
Table 2.
Brain areas with statistically significant altered effective connectivity with right and left pulvinar in subjects with general social anxiety disorder compared with healthy controls.
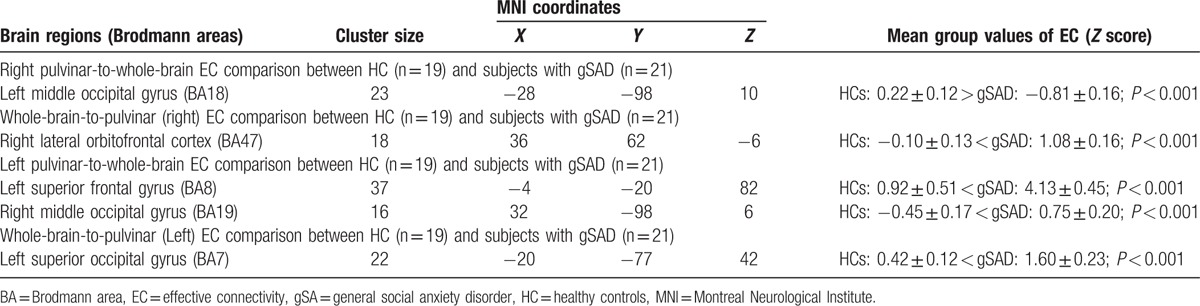
Figure 1.
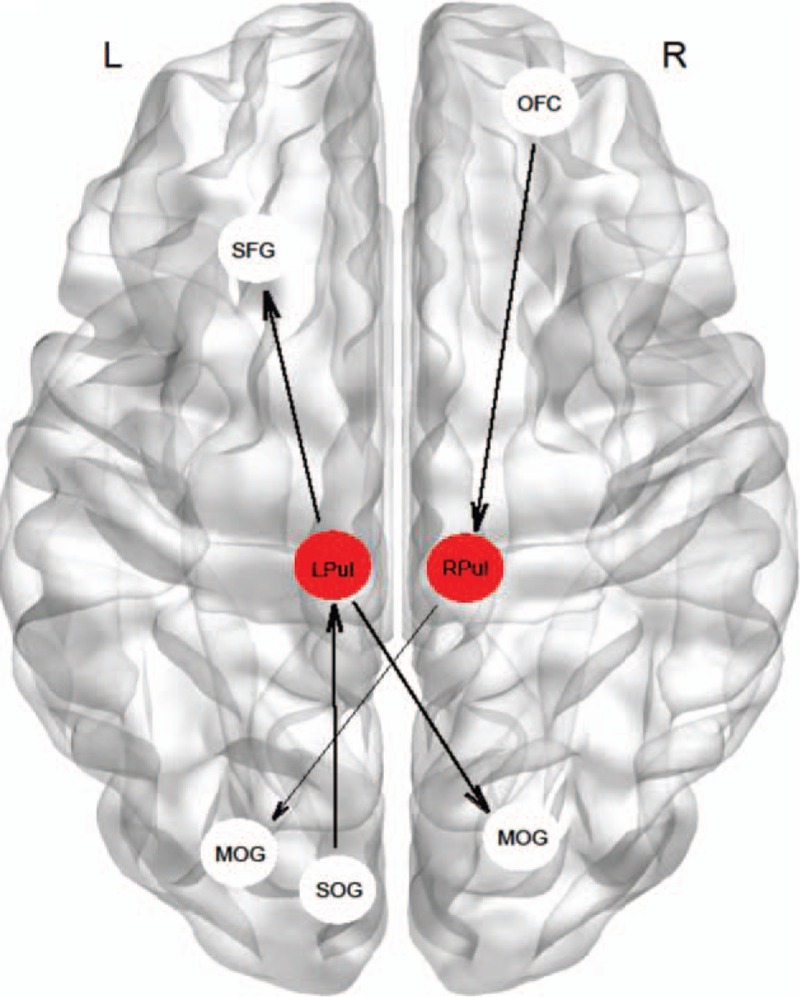
Schematic illustration of altered pulvinar causal outflow and inflow in subjects with social anxiety compared with control group. Direction of causal influences is shown with arrows. Note that all shown connectivity between pulvinar and significant clusters are positive (excitatory). Thicker and thinner lines between nodes represent higher and lower strength of connectivity (causal influence), respectively in gSAD participants compared with healthy controls. L = left, LPul = left pulvinar, MOG = middle occipital gyrus, OFC = orbitofrontal, R = right, RPul = right pulvinar, SFG = superior frontal gyrus, SOG = superior occipital gyrus.
Whole-brain-to-pulvinar EC analysis showed significantly increased causal influence of the right lateral orbitofrontal cortex on the right pulvinar and also significant increased causal influence of the left superior occipital gyrus on the left pulvinar in the gSAD group compared with the HC group (Table 2, Fig. 1).
Furthermore, we measured that the mean GC coefficient values of all the significant group comparison analysis clusters is positive suggesting an “excitatory” causal interaction between pulvinar and those clusters showing significant change in gSAD subjects compared with healthy controls. Our correlation analysis within the gSAD group did not show any significant association between pulvinar EC values and symptom severity (P > 0.05).
4. Discussion
In this preliminary work, we examined pulvinar network function in gSAD and a comparison healthy control group. By applying GC-based effective connectivity analyses of fMRI BOLD signal data collected during an emotional face processing task, we demonstrated an increased pulvinar network interaction with higher order visual processing areas (middle occipital gyrus) and emotion processing and regulatory regions in the frontal cortex (orbitofrontal cortex and superior frontal gyros) in gSAD.
4.1. Thalamus dysfunction in social anxiety
Pulvinar is the largest thalamic nuclei constituting the posterior one-third of the thalamus. Therefore, our findings of altered pulvinar effective connectivity in gSAD relative to controls needs to be discussed in the context of accumulating reports of thalamus involvement in the underlying pathological mechanisms of social anxiety. Several studies have shown abnormal structural, functional, and network connectivity of thalamus in gSAD. Meng et al,[21] reported bilateral lower gray matter density (GMD) in drug-naïve patients with gSAD and a significant positive correlation between age of onset and value of the right thalamus GMD. In a treatment study by Talati et al,[22] it was found that 8-week pharmacological treatment with paroxetine in participants with gSAD resulted in a significant reduction in the right thalamus gray matter volume measured by voxel based morphometry (VBM). Interestingly, they also detected a significant correlation between treatment induced VBM change in the left pulvinar and change in social anxiety symptom severity score. Heightened functional reactivity of thalamus was also reported in several studies in which subjects with gSAD performed emotionally laden visual processing tasks.[23–25] In a resting-state fMRI functional connectivity analysis by Arnold Anteraper et al,[26] hyperconnectivity of thalamus was detected with the precuneus, interior temporal, and parahippocampal regions. Findings of our study expand on the literature of thalamus dysfunction in gSAD by focusing on a more specific anatomical part of thalamus, pulvinar, and applying directed (effective) connectivity method for investigating causal dynamics of involved brain regions that to the best of our knowledge has not been used to investigate thalamus or pulvinar causal network behavior in generalized social anxiety disorder.
4.2. Aberrant pulvinar effectivity connectivity during socio-emotionally salient visual processing in gSAD
Supported by recent intriguing findings of pulvinar functions in cortico-cortical synchronization and its rich anatomical connectivity, particularly the bidirectional connections with different occipital, parietal, insular, cingulate, and frontal regions as well as its unidirectional connection with amygdala, pulvinar is suggested to function as a key node for regulating and integrating information needed for emotionally (and socially)-charged visual processing.[6,8] Our findings of abnormal network interaction of pulvinar with higher level frontal and visual cortex (middle occipital gyrus) suggest a network mechanism for gSAD in which alteration of pulvinar network dynamics may underlie the dysregulation in embedded cortico-subcortical-cortical circuitry of emotionally charged visual processing which contributes to gSAD anxiety symptoms when processing socio-emotional information. Our proposed model can be interpreted as an extension of the standard amygdala-centric “fear circuitry over-activation” neural mechanism of gSAD.
4.3. Pulvinar “overfeeding” as a mechanism of cortical dysfunction in gSAD
GC formulation is based on the concept of temporal precedence: if a signal change (BOLD time series in our case) in B is consistently preceded by a signal change in A, then A Granger-causes B.[19,27] By using this method we found that gSAD patients exhibit increased casual influence of pulvinar on brain regions previously shown to show altered activation in gSAD particularly visual cortex in our case.[24,28–30] We refer to this increased casual influence as pulvinar “overfeeding” and propose a mechanism by which the pulvinar overfeeds or overstimulates the regions which might then be the cause of altered activation in those areas. This serves as a new network based model to help explain how abnormal dynamic interaction between nodes in the related network (emotion-laden visual processing network in this case) can cause or partially be responsible for local dysfunction within nodes of the network.
4.4. Clinical implications
Findings have some important potential clinical implications. In a recently published study by Tadayonnejad et al,[11] it was demonstrated that pharmacological treatment with duloxetine, a serotonergic/noradrenergic agent, can modulate pulvinar effective connectivity strength in subjects with depression. Although this has yet to be tested in patients with gSAD, it suggests such a pharmacological intervention may modulate pulvinar effective connectivity in gSAD as well. Findings also suggest patients with gSAD may benefit from a novel pharmacological or noninvasive neurostimulation techniques like transcranial alternating current stimulation (tACS)[31] that target pulvinar-related networks.
4.5. Methodological consideration
This current work needs to be considered in the context of limitations. We evaluated pulvinar effectivity connectivity in gSAD with a commonly used emotional face processing task, therefore, findings may not generalize to other stimuli. Potentially, stimuli involving social situations[32] or exposure to scrutiny[33] may reveal other currently undetected aspects of pulvinar network dynamics dysfunction in gSAD. Second, a cross-sectional design was used which does not permit inference to state-dependent or trait-dependent findings. Third, BOLD signal has a relatively sluggish nature and the hemodynamic delay between brain areas is variable. Those issues may confound consistent detection of neural-based causal influences between different regions,[34] although, it has been shown that applying GC on real fMRI data results in sensitive and specific identification of causal influences between tested regions.[27] Nevertheless, future studies may want to consider performing GC analysis on higher temporal resolution fMRI data (shorter TR) or using other techniques with significant higher temporal resolution like magnetoencephalography.
5. Conclusions
In this study, we demonstrated that gSAD is associated with aberrant pulvinar networks dynamics (e.g., effective connectivity) manifested in hyperconnectivity in pulvinar-centered cortico-pulvino-cortical networks includes occipital higher order visual processing as well as frontal emotion regulation regions. Our results prompt a broader pathophysiologic model of gSAD that goes beyond fear-based amygdala reactivity to include the pulvinar and its role in processing motivationally relevant social- and emotional information and its effect on frontal and visual/sensory cortices.
Footnotes
Abbreviations: BOLD = blood oxygen level-dependent, EC = effective connectivity, EFMT = emotional face matching task, EPI = echo-planar imaging, fMRI = functional magnetic resonance imaging, GC = Granger causality, GMD = gray matter density, gSAD = general social anxiety disorder, HCs = healthy controls, LSAS = Liebowitz social anxiety scale, OCD = obsessive-compulsive disorder, SPM = statistical parametric mapping, tACS = transcranial alternating current stimulation, TR = time resolution, VBM = voxel based morphometry.
This work was supported in part by National Institute of Health (NIH) National Institute of Mental Health (NIMH) Grant K23MH076198 (PI: K. Luan Phan). RT is supported by NIH NIMH Grant T32MH067631 (PI: Mark Rasenick).
Authors have no conflict of interest to declare.
References
- 1.Stein MB, Stein DJ. Social anxiety disorder. Lancet 2008; 371:1115–1125. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruhl AB, Delsignore A, Komossa K, et al. Neuroimaging in social anxiety disorder: a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev 2014; 47:260–280. [DOI] [PubMed] [Google Scholar]
- 4.Tadayonnejad R, Ajilore O. Brain network dysfunction in late-life depression: a literature review. J Geriatr Psychiatry Neurol 2014; 27:5–12. [DOI] [PubMed] [Google Scholar]
- 5.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ’low road’ to ’many roads’ of evaluating biological significance. Nat Rev Neurosci 2010; 11:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer J, Whitney D. Attention gates visual coding in the human pulvinar. Nat Commun 2012; 3:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padmala S, Lim SL, Pessoa L. Pulvinar and affective significance: responses track moment-to-moment stimulus visibility. Front Hum Neurosci 2010; 4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saalmann YB, Pinsk MA, Wang L, et al. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 2012; 337:753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton JP, Etkin A, Furman DJ, et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 2012; 169:693–703. [DOI] [PubMed] [Google Scholar]
- 11.Tadayonnejad R, Ajilore O, Mickey BJ, et al. Pharmacological modulation of pulvinar resting-state regional oscillations and network dynamics in major depression. Psychiatry Res 2016; 252:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci 2013; 67:311–322. [DOI] [PubMed] [Google Scholar]
- 13.Shaw P, Sharp W, Sudre G, et al. Subcortical and cortical morphological anomalies as an endophenotype in obsessive-compulsive disorder. Mol Psychiatry 2015; 20:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan KL, Coccaro EF, Angstadt M, et al. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biol Psychiatry 2013; 73:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariri AR, Tessitore A, Mattay VS, et al. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage 2002; 17:317–323. [DOI] [PubMed] [Google Scholar]
- 16.Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods 2002; 115:137–143. [DOI] [PubMed] [Google Scholar]
- 17.Thieme Medical Publishers, Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. 1988. [Google Scholar]
- 18.Hamilton JP, Chen G, Thomason ME, et al. Investigating neural primacy in major depressive disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry 2011; 16:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Glen DR, Saad ZS, et al. Vector autoregression, structural equation modeling, and their synthesis in neuroimaging data analysis. Comput Biol Med 2011; 41:1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. NeuroImage 1998; 8:113–128. [DOI] [PubMed] [Google Scholar]
- 21.Meng Y, Lui S, Qiu C, et al. Neuroanatomical deficits in drug-naive adult patients with generalized social anxiety disorder: a voxel-based morphometry study. Psychiatry Res 2013; 214:9–15. [DOI] [PubMed] [Google Scholar]
- 22.Talati A, Pantazatos SP, Hirsch J, et al. A pilot study of gray matter volume changes associated with paroxetine treatment and response in social anxiety disorder. Psychiatry Res 2015; 231:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klumpp H, Angstadt M, Nathan PJ, et al. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: an event-related functional MRI study. Psychiatry Res 2010; 183:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruhl AB, Rufer M, Delsignore A, et al. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res 2011; 1378:72–83. [DOI] [PubMed] [Google Scholar]
- 25.Heitmann CY, Feldker K, Neumeister P, et al. Abnormal brain activation and connectivity to standardized disorder-related visual scenes in social anxiety disorder. Hum Brain Mapp 2016; 37:1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold Anteraper S, Triantafyllou C, Sawyer AT, et al. Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connectivity 2014; 4:81–90. [DOI] [PubMed] [Google Scholar]
- 27.Schippers MB, Renken R, Keysers C. The effect of intra- and inter-subject variability of hemodynamic responses on group level Granger causality analyses. NeuroImage 2011; 57:22–36. [DOI] [PubMed] [Google Scholar]
- 28.Evans KC, Wright CI, Wedig MM, et al. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety 2008; 25:496–505. [DOI] [PubMed] [Google Scholar]
- 29.Gentili C, Gobbini MI, Ricciardi E, et al. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res Bull 2008; 77:286–292. [DOI] [PubMed] [Google Scholar]
- 30.Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology 2005; 52:163–168. [DOI] [PubMed] [Google Scholar]
- 31.Feurra M, Bianco G, Santarnecchi E, et al. Frequency-dependent tuning of the human motor system induced by transcranial oscillatory potentials. J Neurosci 2011; 31:12165–12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakao T, Sanematsu H, Yoshiura T, et al. fMRI of patients with social anxiety disorder during a social situation task. Neuroscience Res 2011; 69:67–72. [DOI] [PubMed] [Google Scholar]
- 33.Gimenez M, Pujol J, Ortiz H, et al. Altered brain functional connectivity in relation to perception of scrutiny in social anxiety disorder. Psychiatry Res 2012; 202:214–223. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Miller KL, Salimi-Khorshidi G, et al. Network modelling methods for FMRI. NeuroImage 2011; 54:875–891. [DOI] [PubMed] [Google Scholar]


