Fig. 3.
Use of injectables (depot medroxyprogesterone acetate, norethisterone enanthate, or unspecified injectable) versus non-use of hormonal contraception and HIV acquisition, among 12 studies considered informative but with important limitations.
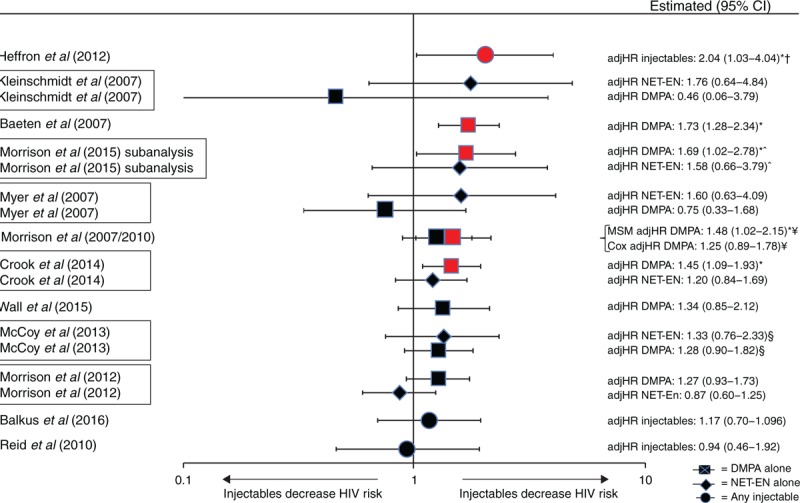
Error bars show 95% confidence intervals. Studies arranged in order of decreasing magnitude of risk estimate, except if a single study disaggregated depot medroxyprogesterone acetate and norethisterone enanthate, in which the case both estimates are adjacent (as indicated by a box around the study identifiers). Graph does not display estimates from marginal structural models, except where use of such models resulted in different conclusion regarding statistical significance; in such cases, estimates from both models are displayed on a single line (also identified by bracket signs). Note: displays all data on injectables (depot medroxyprogesterone acetate, norethisterone enanthate, or unspecified). adjIRR, adjusted incidence risk ratio; adjHR, adjusted hazard ratio. ∗Analysis showed significant findings at P = 0.05 (marker also displayed in red). †Estimate for Cox model taken from slightly updated analysis which controlled for total number of unprotected sex acts. ^Unpublished estimates from a subanalysis of Morrison et al.[26] meta-analysis, restricted to pooled analysis using databases not previously used to publish estimates on hormonal contraceptive methods and HIV acquisition risk. ¥Different statistical models adjusted for slightly different confounders. §Unpublished estimates disaggregated by injectable type.
