Abstract
A key function of the prefrontal cortex is to support inhibitory control over behavior. It is widely believed that this function extends to stopping cognitive processes as well. Consistent with this, mounting evidence establishes the role of the right lateral prefrontal cortex in a clear case of cognitive control: retrieval suppression. Retrieval suppression refers to the ability to intentionally stop the retrieval process that arises when a reminder to a memory appears. Functional imaging data indicates that retrieval suppression involves top-down modulation of hippocampal activity by the dorsolateral prefrontal cortex, but the anatomical pathways supporting this inhibitory modulation remain unclear. Here we bridge this gap by integrating key findings about retrieval suppression observed through functional imaging with a detailed consideration of relevant anatomical pathways observed in non-human primates. Focusing selectively on the potential role of the anterior cingulate cortex, we develop two hypotheses about the pathways mediating interactions between lateral prefrontal cortex and the medial temporal lobes during suppression, and their cellular targets: the entorhinal gating hypothesis, and thalamo-hippocampal modulation via the nucleus reuniens. We hypothesize that whereas entorhinal gating is well situated to stop retrieval proactively, thalamo-hippocampal modulation may interrupt an ongoing act of retrieval reactively. Isolating the pathways that underlie retrieval suppression holds the potential to advance our understanding of a range of psychiatric disorders characterized by persistent intrusive thoughts. More broadly, an anatomical account of retrieval suppression would provide a key model system for understanding inhibitory control over cognition.
Keywords: Retrieval Suppression, Inhibitory Control, Forgetting, Hippocampus, Anterior Cingulate, Nucleus Reuniens
Introduction
Memories, like physical actions, sometimes need to be controlled. For example, although good memory for the past typically is welcomed, this feature poses a problem when memories are unpleasant and intrusive. When people encounter an unwelcome reminder, they strive to limit awareness of the unwanted memory by stopping its retrieval. This retrieval stopping process, known as retrieval suppression, is mediated by an inhibitory control mechanism that suppresses unwanted traces, rendering them less likely to be retrieved in the future (Anderson & Green, 2001; see Anderson & Hanslmayr, 2014, Anderson & Huddleston, 2011 for reviews). Over the last decade, evidence has grown showing that the brain systems underlying retrieval suppression exhibit important similarities and differences to other putative forms of inhibitory control, such as motor response stopping. Like motor stopping, retrieval suppression engages the right lateral prefrontal cortex; but, instead of modulating motor cortical regions, the prefrontal cortex suppresses hippocampal activity that supports retrieval (Anderson et al., 2004; Benoit & Anderson, 2012; Depue, Curran, & Banich, 2007; Depue et al 2015; Gagnepain, Henson, & Anderson, 2014; Levy & Anderson, 2012; Paz-Alonso et al. 2013). These findings suggest that mnemonic functions of the hippocampus are subject to inhibitory control by the prefrontal cortex. If so, retrieval suppression may provide an important model system for studying inhibitory control over thought that complements and generalizes models of inhibitory control based on stopping action.
Whereas the anatomical pathways underlying action stopping are increasingly well characterized (e.g., see, e.g., Schmidt et al., 2013; for a review, see Aron, Robbins & Poldrak, 2014), little is known about how the lateral prefrontal cortex modulates hippocampal activity to suppress retrieval. In this article, we begin to close this gap. In particular, we review anatomical findings observed with non-human primates that inform theories of how the prefrontal cortex could exert inhibitory control over hippocampal activity. In the first section, we describe key brain areas associated with retrieval suppression in human neuroimaging studies, and when they are observed. We then review what is known about interactions between DLPFC and the medial-temporal lobes (MTL) based on primate anatomical studies, and develop candidate pathways that could underlie mnemonic control. Focusing on the anterior cingulate cortex (ACC), we consider in detail the types of neurons to which ACC projects in MTL, and their regional and laminar distribution, with special attention given to their potential to regulate mnemonic activity. After developing candidate pathways, we discuss how well each fits the evidence, and the type of data needed to evaluate these hypotheses.
1. Suppressing Memory Retrieval by Inhibitory Control
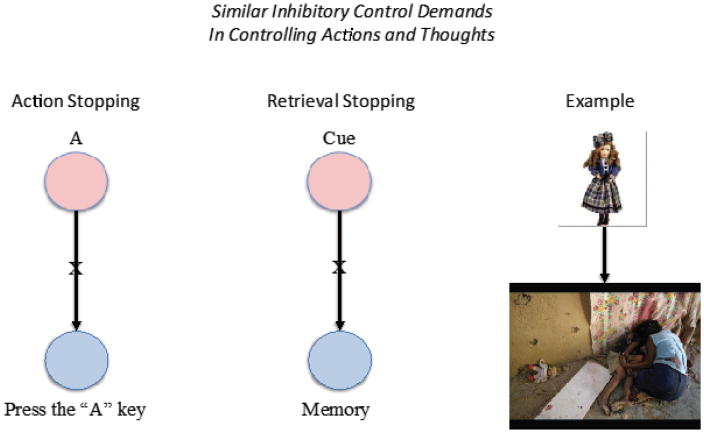
A key premise of this article is that suppressing retrieval builds on prefrontally-mediated inhibitory control mechanisms similar to those engaged to stop motor actions. Consider an example of motor stopping. One evening, the first author accidentally knocked a potted plant off of his window sill. As his hand darted to catch the falling object, he realized that the plant was a cactus. Mere centimeters from it, he stopped himself from catching the cactus. This example illustrates how critical it can be to have the ability to override a strong reflexive response to a stimulus (Fig. 1). Like reflexive motor actions, environmental cues often trigger intrusive memories and thoughts that leap to mind, despite a desire to avoid them. These thoughts can be unpleasant when memories are unwanted. Given the tendency for environmental stimuli to elicit automatic motor or cognitive processes, some mechanism is required that can interrupt both types of processes, if we are to maintain voluntary control over actions and thoughts. Without the capacity to override unwanted processes, we could not adapt behavior or thoughts to changes in our goals or circumstances. The ability to stop is a fundamental function accomplished by inhibitory control, a mechanism believed to suppress representations that drive those processes, enabling the goal-directed interruption of behavior and thought. Of key concern here is how inhibitory control stops episodic memory retrieval when a cue begins to trigger a memory, a situation formally similar to motor stopping (Fig 1).
Figure 1. Stopping actions and thoughts make similar inhibitory control demands.
In a typical motor stopping task (left), a participant might receive a simple cue stimulus and be required to make an associated motor response as quickly as possible (e.g. seeing A and pressing the “A” key). On stop trials, people would be cued, mid-response to withhold the response (symbolized by the “X” on the association between the cue and response). Fulfilling this demands requires inhibitory control to suppress the motor action. Similarly, in a typical retrieval stopping situation, a stimulus appears in the world that is associated to a memory and that will lead us to be automatically reminded of the memory. If a person wishes to avoid being reminded, an inhibitory control process must be engaged to suppress retrieval of the associated memory (symbolized by the X on the association). In real life circumstances, retrieval suppression often arises after a trauma, when people seek to stop being reminded of unpleasant events (right side). For instance, after having witnessed an unpleasant scene (below), a later encounter with an object resembling something from the scene (top) has the power to elicit retrieval of the unpleasant event, triggering the need for control.
1.1 Core Behavioural Findings
Retrieval suppression is often studied with the think/no-think paradigm (hereinafter, the TNT paradigm) (Anderson & Green, 2001). This procedure mimics situations in which we encounter a reminder to a memory we prefer not to think about, and try to keep the memory out of mind. To create reminders, participants study cue–target pairs (e.g., word pairs, or picture pairs; e.g., “ordeal roach”) and are then trained to recall the second item (roach) of the pair whenever they encounter the first (ordeal) as a reminder. Participants then enter the think/no-think (TNT) phase, in which they are asked to exert control over retrieval. On each trial, a reminder from one of the pairs appears in green or red; when the reminder appears in green, participants are to recall the response; but for red reminders, participants are asked to suppress retrieval of the response, preventing it from entering awareness. The latter no-think task asks the participant to override the retrieval process and prevent the associated declarative memory from entering awareness despite the established tendency for the cue to elicit that memory. Participants are told that if the memory does come to mind during no-think trials, they are to suppress it. The key question concerns whether people can recruit inhibition to overcome memory intrusions by learning to prevent the memory from intruding into consciousness, and whether doing so disrupts later retention of the excluded memory. To measure the disruptive aftereffects of retrieval suppression, participants receive a final test in which they are given each reminder and are asked to recall the associated response. Memory performance is compared between items that participants suppressed (No-think trials), items that they retrieved (Think trials), and items that they studied, but neither suppressed nor retrieved during the TNT phase (Baseline trials). Comparing final recall of No-Think items to either Think or Baseline items indicates whether retrieval suppression has a detrimental effect on retention.
The TNT procedure consistently shows that people can stop the retrieval process. This conclusion receives support from several notable effects. First, retrieval suppression abolishes the benefits of reminders on memory, as revealed by the often substantial difference in final retention between Think and No-Think items. Indeed, many studies show that reminders to No-Think items can be presented over a dozen times with little apparent benefit in accessibility of the associated traces. Thus, at a minimum, suppressing retrieval reduces the facilitation that retrieved memories usually enjoy. Second, suppressing retrieval often reduces recall for No-Think items below that observed for Baseline items, a phenomenon known as suppression-induced forgetting. Suppression-induced forgetting is especially informative because it indicates that during retrieval suppression, reminders do not merely fail to enhance retention, they trigger processes that impair voluntary access to the unwanted memory. Third, the impairment of the excluded memory occurs even when it is tested with a novel cue, indicating a generalized impairment of the trace, consistent with the idea that the memory has been inhibited. Most of these effects have been observed with both verbal cue–target pairs and visual pairs such as face–scene pairs, and the effects arise for target items with emotional content (see Anderson & Hanslmayr, 2014, for a review). Thus, stopping unwanted retrievals appears to be achieved in part by suppressing the associated memory, consistent with inhibitory control. As such, the TNT paradigm provides a model for studying inhibitory control over memory that parallels procedures used to study motor response suppression.
Suppression-induced forgetting shows that suppressing retrieval impairs people’s ability to intentionally recall previously suppressed traces. In real world cases of memory control, however, people are rarely motivated to retrieve purposefully the very memories that they have previously suppressed; rather, people are more concerned with stopping the tendency for unwanted memories to intrude involuntarily. A better estimate of the true impact of inhibition on spontaneous retrieval patterns would assess the tendency for memories to come to mind involuntarily, not people’s ability to retrieve them. Research on retrieval suppression indicates that the impact of inhibitory control on involuntary retrievals is even more substantial than its effect on voluntary retrieval. One way that this has been studied is by asking people, after each No-Think trial, whether the unwanted memory came to mind, despite their efforts to stop it from doing so. Remarkably, whereas intrusive memories are extremely common on early suppression trials (often around 60% of trials), they become progressively less common in later suppression trials, showing proportional reductions of nearly 50% (see, e.g., Levy & Anderson, 2012; Benoit, Hulbert, Huddleston, & Anderson, 2015). The effectiveness of reducing involuntary retrievals predicts later suppression-induced forgetting effects, indicating that a common mechanism underlies these phenomena (Levy & Anderson, 2012). These findings suggest that engaging inhibitory control to suppress involuntary retrievals ought to have a substantial impact on spontaneous retrieval patterns in daily life, a possibility consistent with reports of relatively large suppression-induced forgetting effects on free association tests that don’t direct subjects to retrieved suppressed items (Hertel, Large, Stuck, & Levy, 2012).
Given the impact of retrieval suppression on both voluntary and involuntary retrieval, retrieval suppression may provide an important laboratory model of how people control intrusive thoughts in daily life (Kupper, Benoit, Dalgleish, & Anderson, 2014). Intrusive memories and thoughts arise in many clinical conditions such as post-traumatic stress disorder (intrusions), depression (ruminations), attention deficit disorder (distracting thoughts), obsessive/compulsive disorder (obsessive thoughts), schizophrenia (hallucinations), and anxiety (worries). These related symptoms may share a common contributing cause in deficient inhibitory control over memory. Supporting this, adults with attention deficit disorder show impaired suppression-induced forgetting (Depue, Burgess, Willcut, Ruzic, & Banich, 2010), as do participants with post-traumatic stress disorder (Catarino, Kuepper, Werner-Seidler, Dalgleish, & Anderson, 2014) high anxiety (Marzi, Regina, & Righi, 2013), depression (e.g., Joorman, Lemoult, Hertel, & Gotleib, 2009), and ruminative tendencies (e.g., Fawcett et al., 2015). If so, the core intrusive symptoms in these disorders may reflect, in part, compromised function of some aspect of the network underlying inhibitory control over memory. Progress in understanding intrusive symptomatology therefore may benefit from a greater understanding of the anatomical pathways underlying retrieval suppression.
1.2 Core Imaging Findings
Over the last decade, imaging studies have documented the brain systems engaged during retrieval suppression, the areas they modulate, and the dynamic interaction of these regions that produce suppression-induced forgetting. Here we summarize the key role of the prefrontal cortex in retrieval suppression, along with a broader network of areas co-activated with this structure, and the resemblance of this network to that involved in motor stopping. We then describe regions showing reduced activation during suppression, and their potential role as targets of a top-down inhibitory control processes, as evidenced by effective connectivity analyses and relationships to suppression-induced forgetting.
1.2.1 Suppression-Related Activations
Neuroimaging studies have scanned participants during the Think/No-Think phase of the TNT paradigm to isolate the brain systems involved in retrieval suppression. Each trial in this phase presents a cue from a studied pair and only varies by whether participants are cued to retrieve the associated item (Think trials) or to suppress retrieval of the associate (No-Think trials), which is typically signalled by a green or red colored task cue, respectively. Regions more activated during No-Think than Think trials can be assumed to reflect increased engagement of suppression-related task processes above and beyond processes involved in cue processing and retrieval. Studies of retrieval suppression have examined cue-target pairs involving words, face-scene pairs, word-scene pairs, word-face and word-object pairings of both neutral and negative valence (see Anderson & Hanslmayr, 2014, for a review). The observations below indicate generalizations across studies using these varied materials, suggesting broad involvement in retrieval suppression.
1.2.1.1 Right Lateral Prefrontal Cortex
Retrieval suppression engages a strongly right lateralized set of regions within the prefrontal cortex, including dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), posterior middle frontal gyrus (pMFG), and insula. Amongst these, the most spatially extensive activations arise in right DLPFC, which often extend the full anterior-posterior length of the middle frontal gyrus, in a region spanning the border of Brodmann’s areas (BA) 9 and 46 (see, e.g., Fig 2). Posteriorly, this DLPFC region is often spatially distinct from the observed pMFG activation, which occurs in BA 6, raising the possibility that the latter represents a distinct functional activation. Anteriorly, the right DLPFC activation usually extends into the posterior aspect of BA10, bordering 9/46. Indeed, in some studies, DLPFC activations are restricted to this anterior BA9/46/10 area, suggesting that it is a key locus within the DLPFC supporting retrieval suppression. Consistent with this possibility, individual differences in suppression-induced forgetting are often specifically predicted by activation in anterior DLPFC (e.g. Anderson et al., 2004; Depue et al., 2007).
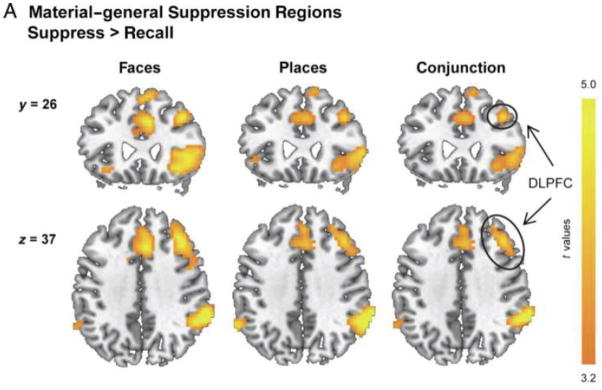
Figure 2. A typical set of suppression-related activations observed in a Think/No-Think study of retrieval suppression.
Benoit et al. (2014) trained people on associations between words and faces, or between words and places. Displayed are brain areas that were significantly more activated when people suppressed (i.e., No-think trials) than when they retrieved items (Think trials), either when they were suppressing faces (left) or places (middle). The right side illustrates the conjunction analyses spanning these materials types, illustrating brain regions that generally are engaged during suppression, irrespective of the particular content. The strong right lateralization of activations is evident, as is the conspicuous involvement of the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), anterior cingulate cortex (ACC) and pre-supplementary motor area pre-SMA).
Several observations support the possibility that anterior DLPFC may be instrumental in implementing a top-down inhibitory control signal that suppresses mnemonic processing. First, this region is particularly engaged by the need to override the retrieval process, as opposed to other strategies that a person might take in preventing an unwanted memory from coming to mind. For example, an alternative approach to suppressing episodic retrieval, would involve a person actively retrieving distracting thoughts that pre-empt or supplant the to-be-avoided memory in awareness. However, Benoit & Anderson (2012) found that controlling retrieval by this type of thought substitution robustly engages left ventrolateral prefrontal cortex regions known to be involved in retrieval. In contrast, instructing participants to not generate thought substitutes, but to instead remain focused on the reminder whilst stopping retrieval altogether, engaged right DLPFC and VLPFC, but not left prefrontal cortex. These findings suggest that right DLPFC is engaged more by the need to suppress the retrieval process. Consistent with this, right DLPFC is more engaged when participants experience an intrusion that needs to be purged during suppression trials, compared to when they do not experience an intrusion (Benoit et al. 2015). Critically, within-subjects comparisons provide evidence for a supramodal inhibition mechanism in right anterior DLPFC, activated during retrieval-suppression, motor inhibition, and emotion regulation (Depue et al. 2015). Finally, as will be discussed, effective connectivity analyses indicate that right anterior DLPFC negatively couples with the hippocampus during retrieval suppression (Benoit & Anderson, 2012; Benoit et al., 2015).
Although we emphasize right DLPFC for the foregoing reasons, it bears emphasis that right VLPFC and bilateral insula activations are regularly observed in studies of retrieval suppression. VLPFC activations tend to arise in right ventral BA44 and 45 (see, e.g., Fig 2), consistent with research on motor response inhibition that has stressed involvement of these regions in inhibitory control over action (Aron, Robbins, & Podrack, 2014). These findings raise the possibility that both VLPFC and DLPFC play critical roles in retrieval suppression. At present, no efforts have sought to distinguish the functional contributions made by these regions. Both may be involved in originating a top-down inhibitory signal; alternatively, left VLPFC activations may primarily reflect increased attentional capture arising when memories intrude into awareness, signalling the need for increased inhibitory control (Corbetta, Patel, & Shulman, 2008). Although the precise functional role of VLPFC cannot yet be discerned, the general pattern of right frontal regions observed during retrieval suppression corresponds well with those observed during motor inhibition, suggesting that these formally similar control demands may engage common systems.
1.2.1.2 Midline Frontal Activations
Across most retrieval suppression studies, regardless of the mnemonic content being suppressed, there are significant activations in frontal midline areas (see Fig 2). These activations include both anterior cingulate cortex as well as the pre-supplementary motor area, and are often, but not always, right lateralized. Sometimes these two regions form part of a single, contiguous activation, but often they appear as distinct activation foci. Within the ACC, the most consistently and robustly engaged region is BA32, though smaller activations in BA24 are often observed. The pre-SMA region falls within the right medial wall, in BA6, extending slightly onto the superior surface of right prefrontal cortex. The medial BA6 pre-SMA region is spatially distinct from the posterior MFG activation in BA6, which is considerably more ventral, and not usually overlapping.
One interesting feature of ACC activations during retrieval suppression is that they occur regardless of the particular strategy people adopt for controlling awareness of an unwanted memory. Thus, whereas attempting to stop the retrieval process entirely engages BA 32, so too does the strategy of thought substitution, in which a participant tries to retrieve alternative memories to supplant the unwanted item in awareness (Benoit & Anderson, 2012). Thus, activation in this region is not diagnostic of retrieval stopping per se. One common feature of these tasks, however, is the presence of conflict, and the need to overcome unwanted activation. In the case of thought substitution, for instance, activation of the ACC may indicate that retrieving a weaker thought substitute instead of the prepotent memory associated to a cue places greater demands on conflict detection and resolution than does retrieving the prepotent response during Think trials (see, e.g., Kuhl, Dudukovic, Kahn, & Wagner, 2007). In the case of direct suppression, the cue also elicits a prepotent memory, which conflicts with the goal of sustaining attention on the cue. Thus, ACC can be viewed as signalling the need for greater control (conflict monitoring), or, instead, as achieving that control in some fashion. We return later to the proposal that the ACC is a key mediator of top-down control in our discussion of anatomical pathways supporting retrieval suppression.
1.2.1.3 Other Activations
Although our primary focus is on lateral and medial prefrontal contributions to memory control, there are additional areas included in the broader network engaged by retrieval suppression. Cortically, retrieval suppression engages regions in the right parietal cortex, including right intraparietal sulcus, along with spatially distinct activations in the supramarginal/angular gyrus (see Fig 2). Similar activations sometimes occur in the left hemisphere, although they are always far smaller in spatial extent and less reliable. Interestingly, these parietal regions bear resemblance to those engaged during motor response inhibition tasks, which are also strongly right lateralized (Levy & Wagner, 2011). More broadly, activation of these particular right parietal areas fits the role of these regions in both voluntary and reflexive orienting of attention (Corbetta et al., 2008), consistent with strong attentional demands made by retrieval suppression. Subcortically, retrieval suppression is associated with greater activity in the basal ganglia, particularly in the right caudate nucleus and putamen (see, e.g., Benoit & Anderson, 2012). As with the parietal cortex, activation in these basal ganglia structures also occurs when people stop prepotent motor responses (e.g., Chambers, Garavan, & Belgrove, 2009; Zandbelt & Vink, 2010), and striatal processes feature prominently in theoretical models of the pathways underlying motor response inhibition (e.g., Wiecki & Frank, 2013). The activation of caudate nucleus and putamen therefore reinforces the similarity of the networks engaged by stopping actions and thoughts, suggesting related mechanisms may mediate these functionally similar demands.
1.2.2 Suppression-Related Reductions
Although the network engaged by stopping retrieval strongly resembles the one involved in stopping actions, the impact of this network appears to differ in each case. Whereas motor response inhibition modulates motor cortical area M1 (see, e.g., Zandbelt & Vink, 2010), retrieval suppression reduces activation in the medial temporal lobes. Generally, brain regions showing significantly less activation during No-Think compared to Think trials are candidates for sites targeted by inhibitory control to stop retrieval. However, negative bold responses need not reflect inhibitory action, and may simply reflect positive engagement during retrieval, and passive lack of recruitment during suppression. Here we briefly review the regions showing negative BOLD responses during retrieval suppression, commenting on the evidence available for inhibitory down-regulation. In general, when BOLD signal in a region is reduced during No-Think trials relative to baseline activity (not merely relative to Think activity), when the affected region shows negative coupling with prefrontal regions implicated in inhibitory control, and when regional BOLD reductions predict forgetting, we suggest that these negative BOLD responses as promising evidence of inhibitory control.
1.2.2.1 Bilateral Hippocampus
Given that established role of the hippocampus in episodic encoding and retrieval, stopping episodic retrieval should reduce activation in this region. Such reductions occur. Activation during No-Think trials is consistently lower than during Think trials in both left and right hippocampi, though this modulation is larger and more consistent in the right than in the left hippocampus, regardless of materials. Though suppression-related reductions have sometimes been observed in the anterior hippocampus, the most consistent reductions arise in posterior hippocampus, a pattern that may prove informative. Both human and animal research points to functional differentiation along the long-axis (anterior to posterior) of the hippocampus (in the rat, ventral to dorsal hippocampus), with differing anatomical features and gene expression and functional connectivity to regions outside the hippocampus (Fanselow & Dong, 2010; Moser & Moser, 1998; Poppenk, Evansmoen, Moscovitch and Nadel, 2013; Strange, Witter, Lein, & Moser, 2014). Differences between anterior and posterior hippocampus have sometimes been attributed to specialization for episodic encoding versus retrieval respectively. This possibility receives support from meta-analyses of functional imaging data (Kim, 2015; Lepage et al., 1998; Spaniol, Davidson, Kim, Han, Moscovitch, & Grady, 2009; however, see Nakamura & Sauvage, 2015), though other theoretical frameworks for this long-axis differentiation have been proposed (see Poppenk et al. 2013, for a review). Given the observed tendency for episodic retrieval to preferentially activate posterior hippocampus, evidence for its reduced activity during suppression is consistent with stopping of retrieval.
On its own, reduced hippocampal activation does not necessarily indicate active down-regulation of hippocampal activity during suppression. Reduced activity during No-Think trials (relative to Think trials) might simply reflect hippocampal engagement during Think trials. Thus, rather than showing that suppression interrupts retrieval, less hippocampal activity may reflect a passive failure to engage retrieval. Evidence has grown, however, that inhibitory control actively reduces hippocampal activation. First, hippocampal activity is also reduced compared to activity during a fixation baseline condition (Benoit & Anderson, 2012; Depue et al., 2007), suggesting that reductions reflect more than just an absence of positive activation during Think trials. Second, DLPFC activation during No-Think trials is often negatively correlated with hippocampal activity (Depue et al., 2010; Depue et al., 2007). Indeed, the magnitude of down-regulation and the correlation with DLPFC has in some studies increased over blocks of the think/no-think phase (Depue et al., 2007), suggesting progressively improved hippocampal regulation with practice. Third, reduced hippocampal activity predicts later suppression-induced forgetting of unwanted memories (Benoit & Anderson 2012; Depue et al., 2007). Finally, effective connectivity analyses show a top-down modulatory influence of DLPFC on the hippocampus (Benoit & Anderson, 2012; Benoit et al., 2015; Gagnepain et al., 2014), with negative coupling from DLPFC predicting both suppression-induced forgetting (Benoit & Anderson, 2012) and reductions in involuntary intrusions over blocks (Benoit et al., 2015). Together, these findings support a role of DLPFC in reducing hippocampal activity, interrupting recollection, and impairing retention.
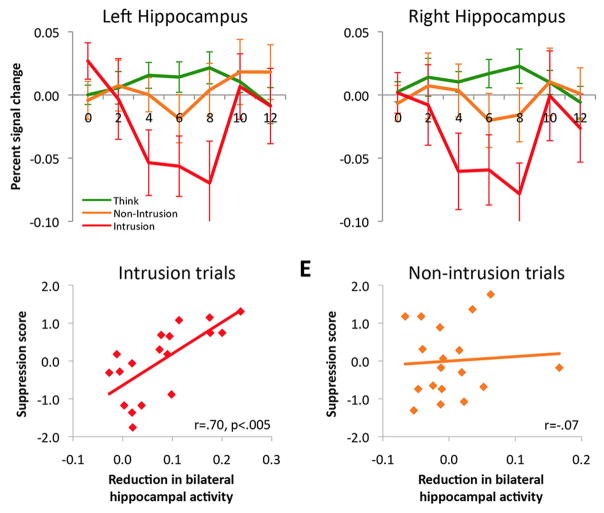
Intrusions of memories into awareness during No-Think trials appear to play an especially important role in triggering down-regulation of hippocampal activity. This point is illustrated by a recent study using phenomenological reports (Levy & Anderson, 2012). To link intrusions to hippocampal regulation, No-Think trials on which an unwanted memory entered participants’ awareness were isolated, and we then linked these intrusions to changes in hippocampal activity. Participants classified their experience after each trial according to whether the cue triggered retrieval of its associated memory. Intrusions elicited strong down-regulation of hippocampal activity (see Figure 3). Although hippocampal down-regulation occurred modestly on non-intrusion trials, the depth of reduction was pronounced during intrusions, when mnemonic awareness needed to be suppressed. Strikingly, the depth of the down-regulation during intrusions strongly predicted suppression-induced forgetting (r = .7). No correlation between down-regulation and forgetting arose, however, during non-intrusions. Strikingly, intrusion-related down-regulations also were associated with more spatially extensive modulation of medial temporal lobe regions, including anterior and posterior hippocampus, entorhinal, perirhinal, and parahippocampal cortices. These findings indicate that higher demands on retrieval stopping may be associated with more extensive regional suppression of mnemonic activity.
Figure 3. Illustration of hippocampal down-regulation during memory intrusions and its relationship to forgetting.
Top Row; Activation in a priori structurally defined hippocampal regions of interest (ROIs) for Think trials and both types of No-Think trials: intrusions where the to-be-avoided memory entered awareness briefly and was purged, and non-intrusions where memory retrieval was successfully stopped. Note that whereas suppression reduces hippocampal activity in general, it does so more robustly for intrusions. Bottom Row, left; The magnitude of signal reduction in the hippocampus during intrusions (the average percentage signal change between 4 and 8 s after stimulus onset, displayed as a positive value) was correlated, across participants, with suppression-induced forgetting of No-Think items on the final test. Bottom row, right: This same measure of hippocampal activity during non-intrusions trials was not related to the amount of suppression-induced forgetting later observed. Error bars for all panels represent SEM.
1.2.2.2 Posterior Perirhinal Cortex and Amygdala
Other medial temporal lobe regions frequently show modulation by retrieval suppression, though the magnitude of modulation depends on the memories being suppressed. For example, posterior perirhinal area 36 is generally modulated by suppression, but modulations also include parahippocampus when the memories are scenes, rather than objects or words (e.g., Benoit et al., 2014). Posterior perirhinal modulation is usually bilateral, whereas parahippocampal modulations for scenes are often right lateralized (e.g., Benoit et al., 2014). Amygdala activity is, in general, only modulated when participants suppress materials with emotional content, although only aversive materials have been studied (Depue et al., 2007; Depue et a., 2010). Some evidence indicates that modulation observed in these regions may be produced, in part, by active down-regulation. For instance, during intrusions, right perirhinal, entorhinal, and parahippocampal cortex shows robust below-baseline activity that predicts suppression-induced forgetting (Levy & Anderson, 2012). Similarly, amygdala activity shows evidence of active reduction (Depue et al., 2007; Depue et al., 2010). However, these suggestions of down-regulation await confirmation with effective connectivity analysis, which would provide more targeted support a role of top-down inhibitory control.
Although effective connectivity evidence has not, as yet, been reported for the perirhinal cortex and the amygdala, other domain-specific cortical regions are actively modulated by DLPFC. For instance, suppressing retrieval of visual objects reduces activity in fusiform gyrus regions involved in visual object perception, and effective connectivity analyses indicate that this modulation is inhibitory (Gagnepain et al., 2014). This finding suggests that, in addition to medial temporal regions, retrieval suppression targets cortical or subcortical regions representing the particular aspect of the content being suppressed. If so, inhibitory modulation of the perirhinal cortex and amygdala seem likely.
1.2.2.3 Other Regions
Although we have emphasized medial temporal regions, other areas associated with episodic retrieval show reduced activity during suppression, compared to retrieval. For instance, reduced BOLD signal is generally found in retrosplenial cortex (BA 29, 30), posterior cingulate cortex (BA 23), left angular gyrus, right frontal polar regions (BA 10), right orbital prefrontal cortex (BA 11), and right ventromedial prefrontal cortex (BA 25, subgenual ACC). In addition, reduced activation is often observed in the basal forebrain region, extending to the medial septal nucleus. Because these modulations are relatively unexplored, little evidence addresses whether they reflect active down-regulation rather than engagement during Think trials. It seems unlikely, however, that every region showing reduced BOLD signal during retrieval suppression is a target of inhibitory control, and that some negative BOLD responses reflect downstream effects arising from successful retrieval stopping. Given the targeted evidence for top-down modulation of hippocampal activity, we focus our anatomical hypotheses on explaining how this phenomenon comes about.
1.2.3 Summary of Core Findings
The foregoing findings underscore the similarity of the networks engaged during motor and memory stopping. These parallels suggest that a broad supramodal process subserves the capacity to override unwanted actions and thoughts (see, e.g., Depue et al., 2015). Nevertheless, evidence suggests that the context in which this mechanism is engaged, and the nature of the material being controlled, alter the coupling of this control process with target regions, allowing control to modulate mnemonic processing rather than motor action. If so, this indicates that the anatomical pathways underlying memory control must be partially distinct from those involved in motor stopping. Next, we consider what those pathways might be, and the nature of the impact that top-down control has on mnemonic processing. First, however, we address broad constraints on models of the role of the prefrontal cortex in inhibitory control, and our perspective on how this function may be achieved.
2. Broad Constraints on the Prefrontal Cortex as a Source of Inhibitory Control
Our view is that the PFC can exercise inhibitory control on representations and processes in general, including on distracting stimuli that impinge on our senses but that are not needed for the task at hand, unwanted motor actions, as well as on complex processes that are the purview of an internal environment—plans, memories and emotions. How does the prefrontal cortex suppress simple sensory signals as well as complex thoughts and memories? What is the circuit basis of inhibitory control, and how might inhibition arise at sites of modulation?
One fundamental constraint on theoretical models of the circuitry of inhibitory control is that they must account for how the prefrontal cortex suppresses activity in distal sites, even though the pathways that link cortices with each other or with subcortical structures are overwhelmingly excitatory in primates (White, 1989). Given this circuitry, how is inhibition achieved? One answer that has received much attention is the idea that excitatory projections from the prefrontal cortex do not directly enact inhibition at all; rather, they enhance to-be-attended (or selected) representations in posterior cortex, and, in doing so, inhibit unwanted competing processes indirectly via local reciprocal inhibitory projections between the target and its competitors. Thus, the prefrontal cortex positively biases a desired process or trace so that it wins a local inhibitory competition with alternative processes. Such biased competition is thought to support selective attention in vision (Desimone & Duncan, 1995) and provides a plausible model of cognitive control (Miller & Cohen, 2001). By this view, the prefrontal cortex does not achieve inhibitory control per se.
An alternative circuit architecture, however, has also been shown to occur: rather than facilitating a chosen target representation, excitatory projections from prefrontal cortex may instead directly excite local inhibitory neurons in the site to be influenced, which then inhibit a distracting stimulus, or unwanted representation or process. For instance, using high resolution methods from the system to the synapse, studies in rhesus monkeys have provided evidence that the prefrontal cortex can exercise inhibitory control when its excitatory pathways leave the cortex, travel in the white matter and innervate inhibitory neurons at the site of termination (Barbas, Medalla, Alade, Suski, Zikopoulos, and Lera, 2005; Germuska, Saha, Fiala, and Barbas, 2006; Medalla, Lera, Feinberg, and Barbas, 2007). Interestingly, these cortical pathways can lead to different types of inhibition with different effects, depending on the specific inhibitory neurons innervated. Mechanisms for inhibitory control at the level of circuits have been discussed elsewhere (Barbas, 2015; Barbas, Bunce, and Medalla, 2013; Barbas and Zikopoulos, 2007). Here we focus on the essential elements of this system for subsequent discussion of plausible hypotheses about the mechanism of inhibitory control for memory.
The ultimate effect of inhibition depends on the type of inhibitory neurons innervated by excitatory pathways. For example, the impact of exciting an inhibitory neuron can vary quantitatively and qualitatively. Quantitatively, inhibition in primates ranges from strong to mild (or modulatory), depending on the type of inhibitory neurons innervated. Qualitatively, excitatory pathways may also form synapses with inhibitory neurons that innervate either other excitatory or other inhibitory neurons, with different functional impacts in each case; in the latter instance, inhibition releases the inhibitory hold on excitatory neurons downstream. Inhibitory neurons in primates can be conveniently classified by expression of three calcium binding proteins, which represent non-overlapping neurochemical classes in primates, including humans (DeFelipe, 1997; Hendry, Jones, Emson, Lawson, Heizmann, and Streit, 1989). Of these classes, interneurons that express the calcium binding protein parvalbumin (PV) innervate perisomatic elements of neurons (DeFelipe, Hendry, and Jones, 1989; Kawaguchi and Kubota, 1997) and thus can exercise strong inhibition at the soma, proximal dendrite or axon initial segment, where impulses are initiated and propagate [see also (Woodruff, McGarry, Vogels, Inan, Anderson, and Yuste, 2011) for the complex effects of a subtype of PV inhibitory neurons]. Another major class includes those that express calbindin (CB), which innervate the dendrites of excitatory neurons, including the apical dendrites of pyramidal neurons (Peters and Sethares, 1997) and thus merely tweak, or modulate their activity. The third class includes inhibitory neurons that express calretinin (CR), which innervate other inhibitory neurons (DeFelipe, Gonzalez-Albo, del Rio, and Elston, 1999; Gonchar and Burkhalter, 1999), at least in the upper layers of cortex (Meskenaite, 1997) and in the hippocampus (Chamberland and Topolnik, 2012). This type of innervation disinhibits excitatory neurons, which are then free to exert excitatory effects elsewhere.
In our hypotheses about inhibitory control over memory, we examine the potential implications of this “direct inhibition” circuit architecture (excitatory pathways innervating inhibitory interneurons) rather than focusing on biased competition. Based on the foregoing precedents about projections and interneuron types, understanding the functional effects of candidate pathways for memory control requires that we differentiate them into excitatory pathways that innervate excitatory neurons, and those that target inhibitory neurons at the site of termination. Moreover, terminating on inhibitory interneurons in and of itself does not allow one to infer functional properties without knowing the type of interneuron affected. In developing hypotheses, we will thus focus on the diversity of inhibition that can be achieved when excitatory prefrontal pathways differentially innervate these broad and functionally distinct neurochemical classes of interneurons (i.e., PV, CB, and CR neurons).
3. Hypotheses about Prefrontal Pathways Supporting Inhibitory Control Over Retrieval
Any hypothesis about the pathways underlying retrieval suppression should honour several constraints. First, the hypothesis should provide a mechanism by which the lateral prefrontal cortex (BA 9/46 in particular) influences activity in the medial temporal lobes, and, in particular, reduces mnemonic activity in the hippocampus. Second, an account should, ideally, explain how retrieval processes can be interrupted in the moment, but also suggest how such acts of control can disrupt later retention. Finally, because the lateral prefrontal cortex does not directly project to the hippocampus, hypothesized fronto-hippocampal interactions must specify one or more intermediate structures through which modulation is achieved.1 These structures must show evidence of being engaged during suppression tasks, and be known to have the capacity to exert inhibitory control in MTL.
The lateral PFC communicates with a large array of cortical and subcortical structures during cognitive tasks. One or more of these structures may serve as an intermediary for communication with the MTL. Some of this communication occurs within the prefrontal cortex itself, between its sub-regions (Barbas and Pandya, 1989; Carmichael and Price, 1996). These intrinsic pathways broadly include robust connections between lateral PFC regions, as well as pathways that link lateral PFC with medial PFC (mPFC, including the ACC), and with the basal prefrontal cortex, commonly called the orbitofrontal cortex. Another group of connections links lateral prefrontal areas associated with working memory (Funahashi, 2006; Fuster, 2008; Goldman-Rakic, 1988) with sensory association and parietal cortices that are engaged during a variety of cognitive tasks (Barbas, 1988; Barbas and Mesulam, 1981; 1985; Medalla and Barbas, 2006; Schall, Morel, King, and Bullier, 1995). Although many of these pathways could support interactions between PFC and MTL in support of retrieval suppression (see, e.g., Depue et al. 2015 for another hypothesis), a full consideration of all potential pathways is beyond the scope of this review. Here we focus selectively on hypotheses in which ACC mediates the influence of lateral prefrontal cortex on memory.
There are excellent reasons to favour the ACC as a candidate region that mediates the inhibitory influence of right DLPFC over MTL during retrieval suppression. Situated on the medial surface as a crescent around the rostral part of the corpus callosum, the ACC, and in particular its area 32 (A32) is well positioned for this function. First, the ACC has unusually strong and diverse connections with the rest of PFC, including area 9/46 in DLPFC (Barbas, Ghashghaei, Dombrowski, and Rempel-Clower, 1999). Thus, the engagement of area 9/46 in imaging studies of retrieval suppression could influence activity in ACC, a possibility that is consistent with existing imaging evidence. Second, due to the ACC’s strong linkage with MTL, the amygdala, hypothalamus and hippocampus (Barbas et al., 1999; Ghashghaei, Hilgetag, and Barbas, 2007; Ongur, An, and Price, 1998; Rempel-Clower and Barbas, 1998), this midline frontal region provides an interface between lateral PFC and structures associated with memory and emotions. Indeed, the connections with structures associated with memory and emotion are strong. Critically, area 32 within the ACC, which is consistently activated during retrieval suppression, has strong connections with MTL cortices, with immediate access to the hippocampus, as elaborated below. Finally, the ACC has specialized connections with motor-related cortices, and especially with motor neurons of the autonomic nervous system that are engaged in emotional arousal (Rempel-Clower and Barbas, 1998). The ACC, in general, is a strong effector system for the emotional motor system, including specific innervation of sites in the amygdala that project to central autonomic structures (Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007). Together, these characteristics position ACC to receive top-down excitatory inputs from the DLPFC and propagate that influence to areas associated with memory and emotion that are targets of suppression.
Theoretical views on the role of the ACC in cognitive tasks are broadly consistent with a potential role in memory control. For example, functional imaging studies in humans have linked activation in the ACC to tasks that feature conflict—when a choice must be made under ambiguous circumstances—and to errors committed in challenging tasks (Botvinick, Braver, Barch, Carter, and Cohen, 2001; Carter, Braver, Barch, Botvinick, Noll, and Cohen, 1998).
Because response conflict (and errors) are arguably ubiquitous features present in many cognitive tasks, this conflict detection function may partially account for ACC’s broad engagement across a variety of tasks. However, it is also clear that the extensive efferent projections of ACC area 32 to other regions, including the MTL, are well-suited to serve more than just a role in conflict detection; rather they are likely to support the enactment of control itself. Indeed, recent computational modelling suggests that the rodent ACC plays a pivotal role in the hierarchical organization of effortful behaviour (Holroyd and McClure, 2015). In the view that we propose here, the ACC’s presumed roles in conflict or error detection are simply subclasses of broader functions that ACC contributes to supporting control during tasks with high cognitive demand.
How might ACC exercise inhibitory control over MTL to suppress retrieval? Here we will consider two main pathways that could support this function: an entorhinal pathway and a thalamic pathway. Before describing our functional hypotheses, however, it is necessary to first discuss broad similarities and differences in how the prefrontal cortex communicates with the medial temporal lobes in primates and rodents.
3.1 Overview of Fronto-MTL Communication in Rodents and Primates
Although the rodent PFC is less differentiated than the primate PFC, a number of common organizational features are observed across species. In general, rodent PFC projections to the MTL follow a similar scheme as observed in macaque monkeys (reviewed in Ongur and Price, 2000; Uylings, Groenewegen, and Kolb, 2003). Anatomical and functional findings of the medial PFC in the rodent suggest that the infralimbic cortices (IL) underlie visceral/autonomic processes similar to the primate ventromedial PFC, whereas the prelimbic cortex (PL) participates in cognitive tasks similar to the lateral PFC of primates (Conde, Maire-Lepoivre, Audinat, and Crepel, 1995; Takagishi and Chiba, 1991; Vertes, 2004). However, the presence of a rodent homologue to the primate DLPFC remains controversial and raises questions of whether this component of an inhibitory control circuit that mediates memory retrieval suppression arises in rats or mice (reviewed in Uylings et al., 2003).
Delineation of other functional prefrontal areas is more consistent across rodents and primates. First, it must be highlighted that key features of ACC connectivity with MTL support the previously hypothesized role of ACC in monitoring on-going behavior in relation to memory of previously learned outcomes (see Botvinick, Cohen, and Carter, 2004; MacDonald, Cohen, Stenger, and Carter, 2000; Rushworth, Behrens, Rudebeck, and Walton, 2007 for reviews). For example, there are robust direct bottom-up projections to the ACC from the hippocampus (Barbas and Blatt, 1995; Cavada, Company, Tejedor, Cruz-Rizzolo, and Reinoso-Suarez, 2000; Insausti and Munoz, 2001; Rosene and Van Hoesen, 1977) that could permit comparison of recently encoded events with expectations based on past experience. Indeed, a growing chorus suggests a role of ACC in integrating existing experiences with novel information to assess on-going events in relation to previously learned knowledge (Euston, Gruber, and McNaughton, 2012; Gonzalez, Kramar, Garagoli, Rossato, Weisstaub, Cammarota, and Medina, 2013; Peters, David, Marcus, and Smith, 2013; van Kesteren, Fernandez, Norris, and Hermans, 2010; reviewed in Wang and Morris, 2010; Wang, Tse, and Morris, 2012; Zeithamova, Dominick, and Preston, 2012).
Importantly, however, the anatomy supports the possibility that ACC’s role is not limited to monitoring, but may also extend to control itself. Although the ACC does not project directly to the hippocampus (Barbas and Blatt, 1995; Cavada et al., 2000; Insausti and Munoz, 2001; Rosene and Van Hoesen, 1977), it does originate parallel projections to a number of memory related structures in the MTL in both the rodent and the primate (Bedwell, Billett, Crofts, MacDonald, and Tinsley, 2015; Delatour and Witter, 2002; Kondo, Saleem, and Price, 2005; Saleem, Kondo, and Price, 2008).2 The rodent infralimbic cortex originates modest projections to the entorhinal and ectorhinal (analogous to perirhinal area 36 in macaque monkeys) cortices, while pathways originating from PL cortex specifically target the entorhinal cortex (Sesack, Deutch, Roth, and Bunney, 1989; Vertes, 2004). A similar series of parallel pathways link the PFC with the MTL cortices in macaque monkeys. Specifically, ACC areas preferentially project to the more medial rhinal areas (28 and 35) and parahippocampal cortices (TH/TF) while posterior orbitofrontal cortex (pOFC) on the basal surface projects to lateral (perirhinal area 36) parts of MTL (Bunce and Barbas, 2011; Bunce, Zikopoulos, Feinberg, and Barbas, 2013; Carmichael and Price, 1995; Kondo, Saleem, and Price, 2003; Kondo et al., 2005; Rempel-Clower and Barbas, 2000; Saleem et al., 2008; Van Hoesen, Pandya, and Butters, 1975).
The trajectories taken by fibers en route between ACC and MTL vary, depending on where they originate. At their origin, ACC fibers are likely part of the cingulum bundle, a large pathway that includes fibers from the cingulate gyrus as well as other cortical and thalamic structures (Mufson and Pandya, 1984) In non-human primates, axons from the mPFC travel in the white matter deep to the medial orbital sulcus and then occupy a position within the external capsule before joining the uncinate fasiculus (Insausti and Amaral, 2008). On the other hand, pathways originating from more dorsal regions of ACC travel through the rostrum of the corpus callosum to the external capsule before entering the uncinate fasciculus (Insausti and Amaral, 2008). Fibers originating from caudal orbitofrontal cortices travel in rostral portions of the uncinate fasciculus, while pathways originating rostrally occupy caudal portions (Insausti and Amaral, 2008). In addition to the ACC, the insula issues a robust projection to the lateral aspect of the entorhinal cortex in the rodent, while a more modest projection links the insula and the entorhinal cortex in monkeys (Burwell and Amaral, 1998; Insausti, Amaral, and Cowan, 1987; Kerr, Agster, Furtak, and Burwell, 2007). Projections from the insula travel in the white matter lateral to the amygdala before entering the white matter deep to the rhinal cortex (Insausti and Amaral, 2008).
Precisely where projections terminate within the MTL depends on where they originate from in PFC. As in the primate, projections from medial and orbital frontal areas in the rodent terminate in all layers of the rhinal cortices, with preferential innervation of some layers depending on the specific site of origin of the pathway (Hoover and Vertes, 2011; Kondo and Witter, 2014). The rodent orbitofrontal projections to rhinal cortices are also organized in a topographic manner, with the lateral orbital area (LO), ventrolateral orbital (VLO), ventral orbital (VO) and medial orbital (MO) areas targeting perirhinal and lateral entorhinal cortex, while medial entorhinal, postrhinal and presubiculum receive inputs mostly from VO alone, which suggests that functionally specialized elements may be present in the circuit (Kondo and Witter, 2014).
Taken together, the above findings support the idea that similar pathways link the PFC with memory related cortices in the medial temporal lobe in both rodents and primates, and ultimately affect the cortical gateway to the hippocampus. It has recently been proposed that the interaction between the mPFC and the entorhinal cortex may serve as a consolidation network, that is dynamically engaged dependent on the age of the memory (reviewed in Takehara-Nishiuchi, 2014). We hypothesize that the mPFC-MTL pathway can also invoke memory retrieval suppression. To understand how mPFC may exert this effect we first review our broad assumptions about the retrieval process, and specific constraints imposed by how information is sent to and from the hippocampus (Burgess et al., 2001; Ektstrom and Bookheimer, 2007; Lehn, et al., 2009; Kesner and Rolls, 2015).
3.2 Overview of Retrieval and Information Flow Within the MTL
Our view of memory retrieval makes assumptions that are worth describing explicitly. First, we assume that retrieval begins with cues that provide partial information about an experience, and that may be perceived in the environment. Retrieval is, then, a progression from this partial information to a completed trace. When cues are perceived, sensory regions process the stimulus and transmit information to the medial temporal lobes and ultimately to the hippocampus. We assume that this cue input triggers pattern completion in the hippocampus, eliciting the remainder of the stored pattern representing the event, which we assume was formed at encoding (Eichenbaum, Yonelinas, & Ranganath, 2007; Bartsch et a., 2011; Rugg & Vilberg, 2013). In the context of the TNT task, providing a reminder elicits the remainder of the association via hippocampal pattern completion processes, including the response item. Upon pattern completion, the products of retrieval trigger outputs from the hippocampus that ascend to neocortex, driving reinstatement of neocortical patterns present at encoding. These neocortical patterns represent the aspects of the sensory experiences of which the event is composed, and their reinstatement at retrieval contributes to creating the experience of recollection (Danker & Anderson, 2010; Gordon, Rissman, Kiani, & Wagner, 2014). By this view, the hippocampus contributes a bound, integrated representation necessary to recreate a multimodal pattern across multiple neocortical sites. It is possible that over time, the consolidation process could lead to an integrated representation in cortex, although for present purposes, we focus on hippocampus-dependent memories.
Given this broad view of retrieval, it is important to consider precisely how information flows into and within the medial temporal lobes to support this process. Sensory information cascades from early-processing to high-order sensory association cortices and then to MTL cortices, eventually arriving in the superficial layers of the entorhinal area 28 (Blatt, Pandya, and Rosene, 2003; Burwell and Amaral, 1998; Lavenex, Suzuki, and Amaral, 2004; Mohedano-Moriano, Martinez-Marcos, Pro-Sistiaga, Blaizot, Arroyo-Jimenez, Marcos, Artacho-Perula, and Insausti, 2008; Mohedano-Moriano, Pro-Sistiaga, Arroyo-Jimenez, Artacho-Perula, Insausti, Marcos, Cebada-Sanchez, Martinez-Ruiz, Munoz, Blaizot, Martinez-Marcos, Amaral, and Insausti, 2007; Steward and Scoville, 1976; Van Hoesen, Pandya, and Butters, 1972; Wellman and Rockland, 1997). Projection neurons in the upper layers (II–III) of area 28 convey cortical input to the hippocampus, where it is thought most mnemonic associations are made (Andersen, Holmqvist, and Voorhoeve, 1966; Insausti and Amaral, 2008; Suzuki, 2007; Wirth, Yanike, Frank, Smith, Brown, and Suzuki, 2003). In contrast, deep layer (V–VI) entorhinal neurons receive hippocampal output and send projections to the neocortex where components of memories are putatively stored in a distributed network (Burwell and Amaral, 1998; Lavenex, Suzuki, and Amaral, 2002; Munoz and Insausti, 2005; Swanson and Kohler, 1986). Signals involved in cued retrieval putatively follow a similar pathway, wherein sensory input enters the hippocampus via rhinal cortices, and drives pattern completion processes that retrieve other aspects of the event not presented in the cue stimulus. At retrieval, hippocampal output is propagated to neocortical areas involved in the original experience, creating synchronous activity thought to underlie the experience of recollection.
Although the foregoing architecture suggests a ready flow of input from sensation to memory-related processing, the passage of information through MTL cortices--both to and from the hippocampus--is not a passive, automatic process. Indeed, sensory information arriving in the superficial layers of the rhinal cortices must overcome a robust local “wall of inhibition” to gain access to the hippocampus (Biella, Uva, and de Curtis, 2002; de Curtis and Pare, 2004). This hypothesis is supported by findings that signals arriving in area 28 are, strikingly, only propagated to the hippocampus with low probability (Pelletier, Apergis, and Pare, 2004), effectively gating the memory circuit. What then determines whether cortical input can overcome this gate, and proceed to the hippocampus? Physiological studies have demonstrated that propagation of signals within the rhinal cortices and onwards to the hippocampus depends on the synergistic effects of activity within the MTL circuit, synergies which are mediated in part by activity in the deep layers of the entorhinal cortex (Kajiwara, Takashima, Mimura, Witter, and Iijima, 2003; Koganezawa, Taguchi, Tominaga, Ohara, Tsutsui, Witter, and Iijima, 2008). For instance, whether inputs to perirhinal cortex are propagated forward can be determined by convergent and synergistic input into the deep layers. These findings demonstrate that the deep layers of the rhinal cortices are integral to information transfer both to and from the hippocampus. Below we discuss how the PFC may impinge on the MTL mnemonic network by exploiting these characteristics.
3.3 The Entorhinal Gating Hypothesis
Our first hypothesis focuses on how ACC might affect information flow into and out of the hippocampus by modulating activity in entorhinal cortex (Figure 4). As reviewed above, prefrontal pathways terminate in the upper and deep layers of the MTL mnemonic cortices where they target both excitatory and inhibitory postsynaptic targets (Apergis-Schoute, Pinto, and Pare, 2006). While the majority of ACC synaptic contacts in the rhinal cortices are with excitatory neurons, a significant number of synapses are made with inhibitory neurons. In the ACC area 32 pathway, synapses with inhibitory neurons are made preferentially with the powerful parvalbumin (PV) neurons in the deep layers of rhinal cortices (Bunce et al., 2013). The deep layers of the entorhinal cortex give rise to two pathways that we hypothesize mediate processes underlying memory retrieval suppression, via ACC interactions with PV neurons.
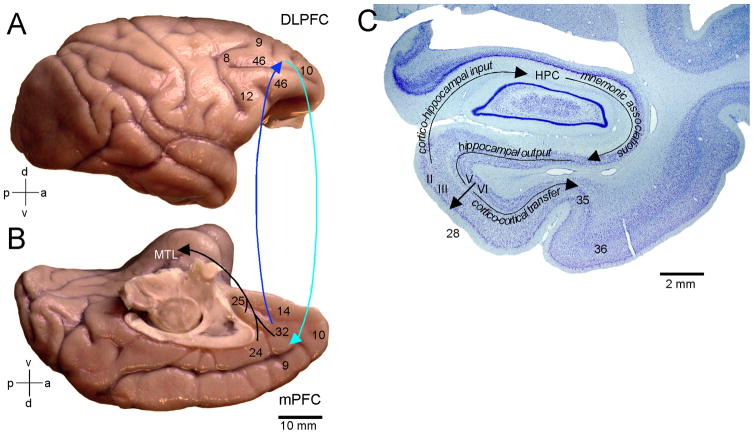
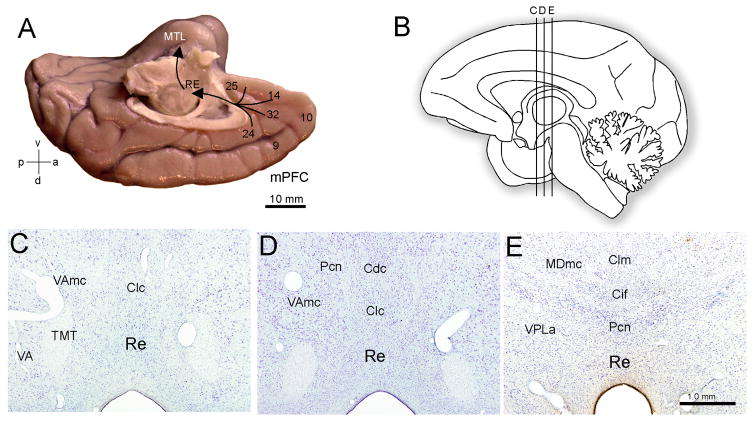
Figure 4. Pathways linking the lateral and medial prefrontal cortices with the medial temporal lobe (MTL) memory system.
A; Lateral surface of the rhesus monkey (Macaca mulatta) brain shows the location of Brodmann’s areas 9 (lateral), 46, frontopolar area 10 and areas 8 and 12. B; Medial surface of the brain shows the medial extent of areas 9 and 10, cingulate areas 24 and 32, and ventromedial areas 14 and 25. Lateral and medial prefrontal areas have robust bidirectional connections (cyan and blue arrows). The predominant projection to the medial temporal lobe (MTL) originates from the medial prefrontal areas in the anterior cingulate and terminates in the entorhinal (area 28) and perirhinal (area 35) cortices. C: Nissl stained coronal section through MTL of the monkey. Area 28 upper layers (II–III) originate the predominant cortical input to the hippocampus (HPC) where it is thought most mnemonic associations are made and contextually driven retrieval occurs. Hippocampal output first reaches the entorhinal deep layers (V–VI), which originate an ascending projection to the upper layers serving as a point by which signals can re-enter the hippocampal loop. Additionally, entorhinal deep layers originate the majority of cortico-cortical projections underlying the transfer of hippocampal signals to the perirhinal (areas 35, 36) and parahippocampal cortices (not shown).
The first entorhinal pathway directs projections to neocortical areas and likely transfers emergent hippocampal output through the rhinal and parahippocampal cortices to the rest of the neocortex, supporting reinstatement of cortical processing during retrieval. ACC is positioned to influence output transmission through this pathway in two ways. On the one hand, transmission can be enhanced by excitatory attentional signals from mPFC, which can facilitate the transfer of signals from the entorhinal to the perirhinal cortices (Paz, Bauer, and Pare, 2007). On the other hand, ACC can suppress this transmission via its innervation of PV inhibitory neurons, impeding the output necessary to create neocortical activity underlying recollection. Thus, ACC can suppress reinstatement of perceptual traces, possibly controlling the extent to which people re-experience the sensory aspects of an event.
By innervating PV neurons the ACC can do more than controlling hippocampal output, however. ACC input to PV interneurons in the deep layers should also suppress perceptual input into the hippocampus needed for cue-driven retrieval. There are strong indirect pathways from neocortices to the hippocampus; entorhinal cortex receives, directly or through a series of connections, robust projections from high-order sensory association areas in temporal cortex (Mohedano-Moriano et al., 2008; Van Hoesen and Pandya, 1975a; b). These pathways originate from well-laminated temporal sensory association cortices and ultimately innervate the entorhinal cortex, which has by comparison a simpler laminar structure and lacks a granular layer IV. These pathways innervate all layers of entorhinal cortex but they show a bias for the deep layers. The laminar pattern of connections that emanate mostly from the upper layers of well-laminated areas and target strongly the deep layers of areas with simpler laminar structure, is consistent with the rules of the structural model for cortico-cortical connections (Barbas and Rempel-Clower, 1997). Critically, many of these higher order sensory cortical inputs to the deep layers of entorhinal cortex will ultimately propagate to upper layers of entorhinal cortex, a key region from which most cortical inputs to the hippocampus originate. ACC innervation of the powerful PV inhibitory neurons in the deep layers is positioned to suppress this ascending input, suggesting a circuit mechanism through which unwanted cue input may be filtered out.
One final effect of ACC input to PV neurons in the deep layers is to alter the ability of hippocampal outputs themselves to feed back into input pathways of the hippocampus and synergize with that input. This feedback process may arise via a second entorhinal pathway that ascends from its deep to its upper layers (Buckmaster, Alonso, Canfield, and Amaral, 2004; Kloosterman, van Haeften, Witter, and Lopes da Silva, 2003; Kloosterman, Witter, and Van Haeften, 2003; van Haeften, Baks-te-Bulte, Goede, Wouterlood, and Witter, 2003). As noted above, most projections from cortex to the hippocampus originate in the upper layers of the entorhinal cortex, providing a key input pathway. Interestingly, the ascending entorhinal pathway allows newly emergent hippocampal output to the deep layers to re-enter the hippocampal loop. Ordinarily, this ascending hippocampal output may converge with sensory inputs to the upper layers, synergizing with it. Because signal propagation from cortex to the hippocampus often critically depends on synergistic inputs mediated by the deep layers (Kajiwara et al., 2003; Koganezawa et al., 2008), this ascending feedback may enable coordinated neural activity that enables inputs to overcome the rhinal wall of inhibition. Critically, suppressing activity in the deep layers via PV interneurons should also reduce this synergistic effect, further gating the hippocampus from cortical information (Figure 5).
Figure 5. Proposed ACC-Rhinal circuit underlying entorhinal gating.
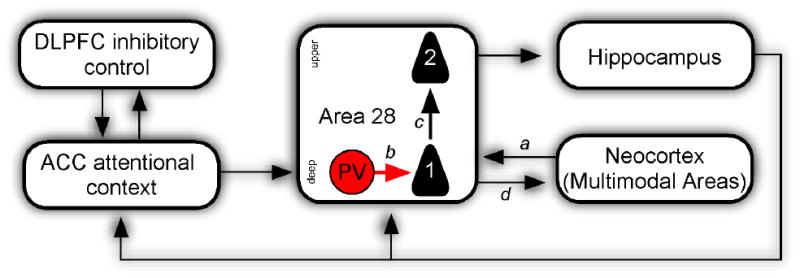
The diagram is simplified and shows only key pathways pertinent to inhibitory control. Lateral prefrontal cortex (DLPFC) shares bidirectional connections with medial prefrontal cortex (mPFC) in the anterior cingulate cortex (ACC), which is poised to exert inhibitory control on downstream structures in the medial temporal lobe (MTL). The ACC pathway to MTL forms synapses with excitatory neurons (not shown) as well as with parvalbumin (PV; red) putative inhibitory neurons in the deep layers of rhinal cortex (area 28). Through innervation of the powerful PV inhibitory neurons, the ACC may inhibit output of and input to the hippocampus. In the latter case, PV neurons would inhibit inputs to the ascending pathway (1) to the upper layers of area 28 (2), resulting in a loss of coordinated neural activity necessary to overcome rhinal inhibition, gating the hippocampus from incoming cortical information of sensory cues arriving from neocortical multimodal areas (a).
This entorhinal gating mechanism, especially the gating of cue input, may account for the hippocampal and perirhinal quiescence observed during memory retrieval suppression and could serve the functional processes by which memory retrieval is suspended (for a related discussion, see Depue, 2012). Related to this, previous intracranial recording studies in patients with epilepsy have proposed that the frontal cortices can modulate hippocampal encoding mechanisms via effects on the rhinal cortices (Ludowig, Moller, Bien, Munte, Elger, and Rosburg, 2010).
3.4 The Thalamo-Hippocampal Modulation Hypothesis
In our first hypothesis, we emphasized how the ACC may indirectly affect hippocampal retrieval processes via strong innervation of inhibitory cells in the entorhinal cortex. In our second proposed pathway, we consider the possibility that ACC may modulate hippocampal processes actively, not merely by gating input. One pathway by which this type of modulation may be achieved is via robust bidirectional connections with the thalamic reuniens nucleus (RE). The RE is notable because it originates one of the principal thalamic inputs to the MTL. In the monkey, many PFC regions share connections with midline nuclei of the thalamus, including the reuniens, but the strongest connections of RE are with ACC areas in mPFC (Barbas, Henion, and Dermon, 1991; Dermon and Barbas, 1994) (see Figure 6).
Figure 6. Proposed ACC-thalamic nucleus reuniens (RE) pathway underlying hippocampal modulation.
A. The medial prefrontal cortex (mPFC) areas that receive robust projections from the hippocampus (HPC) (in the anterior cingulate cortex region) send a pathway to the RE that, in turn, originates one of the most prominent thalamic pathways to the medial temporal lobe (MTL), which terminate in CA1 as well as the subicular and rhinal cortices. B; Midsagittal drawing of the human brain denoting the position of three rostrocaudal levels through the RE which correspond to the three Nissl stained coronal sections (C, D, E).
The pathway through the reuniens provides an important candidate mechanism through which PFC could impact hippocampal processing. In rats, reuniens pathways terminate along the entire septotemporal (dorsoventral) extent of CA1 and the subicular cortices as well as all layers of the ecto-, peri- and entorhinal cortices (Bertram and Zhang, 1999; Cassel, Pereira de Vasconcelos, Loureiro, Cholvin, Dalrymple-Alford, and Vertes, 2013; Dolleman-Van der Weel, Lopes da Silva, and Witter, 1997; Herkenham, 1978; McKenna and Vertes, 2004; Segal, 1977; Varela, Kumar, Yang, and Wilson, 2014; Vertes, 2006; Vertes, Hoover, Szigeti-Buck, and Leranth, 2007). MTL areas that receive RE input generally issue return projections back to the nucleus, which arise from the deep layers (McKenna and Vertes, 2004; Vertes, Hoover, Do Valle, Sherman, and Rodriguez, 2006). In the rodent hippocampus, RE projections terminate in stratum lacunosum moleculare and synapse on the spines of principal neurons and dendritic shafts, which are thought to include a significant number of inhibitory targets including chandelier, basket, lacunosum-moleculare interneurons and interneurons located at the radiatum-lacunosum moleculare border (Bokor, Csaki, Kocsis, and Kiss, 2002; Dolleman-Van der Weel et al., 1997; Dolleman-Van der Weel and Witter, 2000; Herkenham, 1978; Wouterlood, Jorritsma-Byham, and Goede, 1990). Inhibitory neurons in the hippocampus are neurochemically diverse with different populations in stratum lacunosum-moleculare (SLM) expressing vasoactive intestinal peptide (VIP) and calbindin (CB) and those neurons in radiatum bordering SLM express cholecystokinin (CCK) or calretinin (CR) (Freund and Buzsaki, 1996), see Fig. 6.
Thus, similar to PFC-rhinal interactions, ACC signals are positioned to affect hippocampal dynamics via RE interactions with distinct inhibitory and excitatory post-synaptic targets. Such an influence should affect synchronous activity between the structures, disruptions of which have known functional consequences including working memory deficits (Duan, Varela, Zhang, Shen, Xiong, Wilson, and Lisman, 2015; Griffin, 2015). Connections between midline thalamic nuclei and distributed limbic structures are proposed to play a role in awareness and arousal mediating information transfer between the mPFC and the hippocampal formation (HCF; comprised of the hippocampus as well as the subicular, rhinal and parahippocampal cortices of the MTL (Cassel and Pereira de Vasconcelos, 2015; Van Der Werf, Jolles, Witter, and Uylings, 2003; Vertes, Linley, and Hoover, 2015). Importantly, recent evidence suggests that projections from ACC to RE play a role in modulating excitability of hippocampal neurons, thereby controlling the specificity with which memories are encoded. Alterations to RE-hippocampal interactions influence the tendency to overgeneralize fear memories to novel contexts in which fearful events did not happen (Ito, Zhang, Witter, Moser, and Moser, 2015; Xu and Sudhof, 2013), a tendency that may be relevant to contextually inappropriate recall of traumatic flashback memories.
Much of the current work on interactions between ACC, RE, and MTL has focused on the potential of this circuit to transmit information between the prefrontal cortex and the hippocampus (Ito, Zhang, Witter, Moser, & Moser, 2015), or to positively modulate the state of the hippocampus at encoding, when greater memory specificity is needed (Xu & Sudhof, 2013). The existence of substantial projections from RE to inhibitory interneurons in CA1, however, raises a functional possibility that has not yet been adequately considered—that this circuit may also, in some contexts, suppress hippocampal processing. When and how RE’s engages excitatory or inhibitory influence on hippocampal activity via its projections to excitatory and inhibitory interneurons needs to be explored. Here we speculate that the presence of projections to inhibitory interneurons in CA1 could support a negative modulation of hippocampal activity in service of inhibitory control over retrieval. Notably, this influence could be widespread throughout MTL, affecting the hippocampus as well as entorhinal and perirhinal cortices.
4. Relation of the Hypotheses to Human Data on Retrieval Suppression
Because the forgoing pathways and their targets in the medial temporal lobes have not been characterized in detail in humans, our hypotheses are necessarily speculative. Nevertheless, we can ascertain how well our hypotheses agree with regular patterns of activity in imaging studies, and what aspects of the data can be explained. In this section, we consider the strengths and weaknesses of our hypotheses in relation to the data presented at the outset, and highlight missing data that would be helpful in addressing them.
4.1 Evidence for the Entorhinal Gating Hypothesis
Several aspects of this hypothesis fit remarkably well with imaging activations. First, the hypothesis accommodates the increased activations observed in both anterior DLPFC 9/46/10 and ACC area 32, which would be expected if this pathway were engaged more during suppression than retrieval. To the extent that these regions are homologous across primates and humans, the match of the observed activations to particular subregions within the DLPFC and ACC to elements of this pathway is especially encouraging. The right lateralization in humans would have no ready explanation, however, based on primate anatomy alone.
Second, the hypothesis can explain reductions in mnemonic activity in the medial temporal lobes. If top-down inputs from ACC to entorhinal cortex drive inhibitory activity, cellular activity that would ordinarily support the transmission of information into and out of the hippocampus would be reduced. This may lead to reductions in BOLD signal in the entorhinal cortex as well as downstream areas that would ordinarily be driven by this activity. In essence, the prefrontal cortex would gate inputs into and out of the hippocampus, which may lead to a relative quiescence of activity in the latter. To the extent that hippocampal retrieval processes rely on driving input arriving through the entorhinal cortex, pattern completion would not occur and recollection would be pre-empted. If entorhinal inhibition instead suppressed output from the hippocampus, rather than input into it, hippocampal pattern completion may happen, but the products of this process could not drive synchronous activity with neocortical sites that represent the content of the event, which may pre-empt the recollection experience. This latter output gating possibility, by itself, would not, without additional assumptions, explain reduced hippocampal activity that is typically observed, because cues would be expected to drive hippocampal activity in the normal fashion; it could, however, account for reductions in BOLD signal in downstream components of the network of recollection-related regions privy to hippocampal output, such as retrosplenial cortex, posterior cingulate cortex, and left angular gyrus (Rugg & Vilberg, 2013).
Some aspects of the entorhinal gating hypothesis seem discrepant with details of patterns observed in imaging data. For instance, one might expect to observe significant reductions in entorhinal activity during suppression, but this has generally not been reported. Rather, BOLD reductions are more often reported in the hippocampus and in the posterior perirhinal/parhippocampal cortex, rather than in the entorhinal cortex. Relatedly, if entorhinal gating were the main mechanism driving hippocampl reductions, it is unclear why the most reliable reductions in BOLD signal would arise in posterior, rather than anterior, hippocampus. Nevertheless, most studies of retrieval suppression have not carefully scrutinized the localization of activations within the MTL to determine whether, in addition to hippocampus, other MTL regions are affected. The one study that has done this (Levy & Anderson, 2012) did find evidence for entorhinal, perirhinal, and parahippocampal down-regulation, especially in the right hemisphere, and, with the exception of perirhinal modulation, exclusively in response to memory intrusions. Moreover, until a quantitative meta-analysis is performed on medial temporal activations, it is prudent to reserve judgment on whether details of MTL reductions agree with this hypothesis.
The entorhinal gating hypothesis is best positioned to explain the momentary regulation of conscious recollection, for the duration of time that suppression is being enacted. Once retrieval suppression ends, gating should be reduced and the transmission of information between neocortex and the hippocampus may resume as normal. It is possible, however, that sustained inhibitory inputs to the entorhinal cortex may induce persisting effects that disrupt encodings needed for future retrieval attempts, or—instead—lead to new inhibitory learning that interferes with later retrievals. Thus, depending on whether sustained inhibition triggers persisting effects on entorhinal representations, this hypothesis could account for memory deficits arising from suppression. At a minimum, entorhinal gating is a strong candidate for explaining how mnemonic awareness can be pre-empted proactively via input gating.
Because the anatomy of this pathway suggests an entorhinal site of inhibition, evidence suggesting active modulation of the hippocampus may not be as readily explained by this mechanism. For instance, effective connectivity analyses indicate an active top-down modulation of hippocampal activity that contributes to later suppression-induced forgetting (Benoit & Anderson, 2012; Benoit et al; 2014; Gagnepain et al. 2014). Moreover, hippocampal activation during memory intrusions shows especially robust down-regulation, relative to non-intrusions, which strongly predicts later forgetting (Levy & Anderson, 2012). It’s not clear how gating input into the hippocampus would produce these observations. It is possible, however, that the inhibitory influence of ACC on entorhinal cortex may be propagated into the hippocampus. At present, however, no direct anatomical basis for such propagation has been established or even sought, so this possibility must remain speculative.
The viability of the entorhinal gating hypothesis of retrieval suppression should be scrutinized closely in future work. For example, no study has yet examined functional or effective connectivity of ACC during retrieval suppression, to determine whether it (a) positively couples with DLPFC during suppression, and (b) negatively couples with structures within the medial temporal lobes. This hypothesis predicts that the established influence of the DLPFC on the medial temporal lobes should be mediated primarily by BA32. Second, greater attention should be devoted to characterizing the precise pattern of modulation within subregions of the medial temporal lobes, to firmly establish the involvement of entorhinal modulation. Finally, if entorhinal modulation occurs, it is important to explore how such modulation influences activity within the hippocampus, and whether suppression affects inputs into, outputs from, or processing within this structure.
4.2 Evidence for the Thalamo-Hippocampal Modulation Hypothesis
The idea that the hippocampus may be modulated via the reuniens shares many strengths with the entorhinal gating hypothesis because it also presupposes that the ACC is the pathway by which DLPFC influences mnemonic activity. An additional strength, however, is that the reuniens pathway provides a mechanism of inhibitory action capable of influencing hippocampal activity. Because a substantial fraction of projections from the reuniens terminate on GABAergic inhibitory neurons in CA1 (Dolleman-Van der Weel et al., 1997), this pathway would explain reductions in hippocampal activity in terms of direct inhibitory action, rather than by input gating. It is possible, therefore for ACC modulation of the nucleus reuniens to provide a modulatory influence over hippocampal functions. This hypothesis is appealing because it converges with recent evidence in rodents for a role of interactions between the prefrontal cortex and nucleus reuniens in modulating hippocampal activity during encoding, determining the specificity at which events are encoded. If such an interaction occurs in primates, and can be engaged to control episodic retrieval, this hypothesis would link inhibitory control over memory with work in the rodent literature concerning hippocampal modulation. Additionally, through its extensive network of pathways targeting the hippocampus as well as the entorhinal and perirhinal cortices, the RE may serve a more global modulatory role in the MTL (McKenna and Vertes, 2004; Vertes et al., 2006).
There are several shortcomings to the reuniens hypothesis, however. First, although the reuniens and its projections to the hippocampus have been studied in rodents, the corresponding anatomy has not been well characterized in primates. It is possible that the characteristics of this pathway may be quite different across species. Particularly lacking is an indepth characterization of the types of inhibitory neurons that reuniens innervate, and their likely impact on mnemonic processes. Second, few studies of retrieval suppression have reported thalamic activations, and those that have, have reported activations in the pulvinar nucleus (Depue et al. 2007). If the thalamus were a major intermediary structure in achieving retrieval suppression, one might expect reports to be more common. However, there are good reasons to be cautious about interpreting this lack of activation too strongly. The nucleus reuniens is a small and not often studied structure located in the midline of the ventral thalamus. It is possible that activations of this structure could have been missed in reporting of activation peaks, especially given its small spatial extent (which may not have exceeded imaging spatial extent thresholds) and given that it might not have played a key role in hypotheses about retrieval suppression. A more focused analysis of thalamic activations during retrieval suppression is therefore required before the role of the nucleus reuniens in retrieval suppression can be evaluated.
As with the entorhinal gating hypothesis, the viability of the reuniens pathway needs to be scrutinized with focused analyses that test key predictions. Evidence for modulation of activity in this nucleus should be evaluated in existing and future studies of retrieval suppression. How does activation in reuniens vary across retrieval and suppression conditions, if at all? If differential activation is observed, can functional or effective connectivity be established with the ACC? If the reuniens triggers suppression of hippocampal activity or MTL activity generally, functional connectivity should reveal differential coupling with the hippocampus across the think and no-think conditions. Ideally, this coupling should predict differences in forgetting of suppressed items. Finally, the behavioural impact of inhibitory inputs to the hippocampus from RE could be investigated in rodent models, to determine the precise contribution they make to fronto-hippocampal interactions, and whether this is consistent with the proposed role in inhibitory control.
4.3 A Dual Pathway Hypothesis
One final intriguing possibility is that both entorhinal and reuniens pathways may contribute to controlling memory retrieval, but may do so at different temporal stages of the retrieval process, under different conditions. For instance, if modulating entorhinal activity gates input into the hippocampus, it could put a brake on retrieval processes before they begin, by depriving the hippocampus of key driving inputs from neocortical regions involved in processing retrieval cues. As such, it may be an effective means of input gating, wherein control can interrupt the flow of input to the retrieval process. In principle, if applied quickly in response to a reminder, this form of control could prevent a memory from intruding into awareness in response to the reminder, achieving proactive control over awareness. When input gating fails and entorhinal inputs drive hippocampal retrieval processes, it may be necessary to modulate hippocampal activity directly, via the reuniens pathway, to globally suppress unwanted activity in the hippocampus and the other structures to which reuniens projects (e.g., entorhinal and perirhinal cortex). By this view, the reuniens may suppress hippocampal pattern completion processes and disrupt hippocampally dependent traces, whilst also preventing unwanted hippocampal activation from propagating to neocortex. This type of hippocampal suppression may contribute strongly to reactive control.
This division of labor between the pathways could provide a strong explanation of two important observations. First, if engagement of the RE increased when proactive control failed, it could explain why hippocampal down-regulation arises primarily during intrusions, and far less so on non-intrusion trials in which proactive control has been successfully engaged (Levy & Anderson, 2012; figure 3). Second, if RE drives down-regulations during intrusions, it could account for why the spatial extent of down-regulation expands during memory intrusions to include the entire length of the hippocampus, entorhinal, and perirhinal cortices (Levy & Anderson, 2012). This broadened set of regions encompasses many of the MTL regions to which RE projects. This dual pathway hypothesis further makes the distinctive prediction that functional connectivity patterns between the ACC and target structures may differ depending on whether proactive or reactive control occurs.
5. Concluding Remarks
Understanding how people direct their actions and thoughts will ultimately require a neuroanatomical account of the ability to stop, a truly fundamental process of self control. Research on stopping has expanded dramatically over the last decade, but has focused primarily on how organisms stop physical actions. Although this emphasis has been enormously useful, models of inhibitory control based on motor response stopping do not clearly address how people control unwanted thoughts. This cognitive inhibition must, at some level, involve distinct pathways. Here we considered research on retrieval stopping as a model system for cognitive inhibition, with the particular aim of developing hypotheses about the anatomical pathways that could support prefrontal control over the medial temporal lobes. In particular, we focused on the anatomy underlying one class of fronto-medial temporal pathways, via the anterior cingulate cortex, because the characteristics of this pathway have been documented in detail in non-human primates and because projections from the anterior cingulate are known to be involved in other forms of inhibitory control, including the suppression of distracting stimuli in auditory association cortex (Germuska et al., 2006; Medalla and Barbas, 2009; 2010; 2012).
As this review reveals, the ACC (BA 32) is anatomically well positioned to enable the dorsolateral prefrontal cortex to suppress information flow into and out of the hippocampus via robust projections to entorhinal cortex. Studies in non-human primates document excitatory projections from ACC that terminate on several classes of inhibitory interneurons in these structures. Many of these projections terminate on PV inhibitory interneurons, which are known to exert rapid and robust inhibitory influences on the cell bodies of pyramidal cells. These characteristics could support the ability to amplify the gating of information flow both into and out of the hippocampus, preventing cues from driving hippocampal retrieval processes, or, instead, suppressing hippocampal output that would elicit cortical reinstatement of event features necessary for the experience of recollection. Moreover, it is possible, in principle, that the influence of these projections on rhinal cortex may be propagated into the hippocampus itself, providing a basis by which this structure can be modulated by inhibitory control. Parallel to this, ACC area 32 also robustly innervates midline thalamic nuclei such as the nucleus reuniens. The reuniens itself robustly innervates MTL regions spanning hippocampal area CA1, entorhinal, and perirhinal cortices, and its projections synapse on a significant proportion of inhibitory neurons. Recent optogenetic evidence indicates that the medial prefrontal cortex (including ACC) drives activation of the nucleus reuniens to modulate hippocampal state and dictate the level of specificity at which an event is encoded. Thus, precedent exists to suggest that, in addition to gating the input into and output from the hippocampus, ACC is positioned to modulate activity in the hippocampus itself, potentially contributing to memory control effects observed with retrieval suppression.
At present, it remains unclear which of these pathways is the critical mechanism underlying retrieval suppression--or indeed whether an altogether different mechanism may instead provide a more plausible account. Our intention was to consider the wealth of anatomical knowledge available from primate studies to illustrate that clear pathways exist that could support suppression of episodic retrieval processes. In doing so, we hope to encourage neuroscientists to more deeply scrutinize these and other potential pathways of inhibitory control over memory. We believe that isolating the anatomical pathways and mechanisms of action that underlie retrieval suppression holds significant potential to advance our understanding of a range of psychiatric disorders characterized by persistent intrusive thoughts, including post-traumatic stress disorder, schizophrenia, anxiety, depression, and ADHD. More broadly, we believe that an anatomical account of retrieval suppression would provide an important model system for understanding inhibitory control over cognition generally—one that both complements and expands what has been learned by studying inhibitory control of overt behavior.
Acknowledgments
The current research was supported by a grant from the UK Medical Research Council (MC-A060-5PR00) to M.C.A., and grants from NIH (NIMH R01MH057414, and NINDS R01NS024760) to H.B. The authors thank Taylor Schmitz and Yuhua Guo for useful comments on the review of imaging findings.
Footnotes
It is often claimed that the dorsolateral prefrontal cortex projects directly to the hippocampal formation, based on a finding reported by Goldman-Rakic, Selemon, & Schwartz (1984). Goldman-Rakic and colleagues described connections between lateral prefrontal and mostly a medially-situated cortical region that the authors refer to as the caudomedial lobule. Findings by several other investigators (e.g., Insausti and Munoz, 2001; Cavada et al,, 2000; Barbas and Blatt, 1995; Rosene and Van Hoesen, 1977) showed that hippocampal pathways reach primarily the ACC and to a lesser extent the orbitofrontal cortex, which project to the rhinal region (as we have discussed in the review). The HRP injection sites in the Goldman-Rakic et al. study were very large and impinged on the white matter below area 46, reaching as far as the orbitofrontal cortex, which helps explain some of the connections seen in the presubiculum/subiculum. Given these considerations, we regard the Goldman-Rakic conclusion about DLPFC-hippocampal projections as a likely error arising from imprecise injection sites.
A recent paper (Rajasethupathy et al., 2015) reported the existence of a direct projection from ACC to the hippocampus in mice. However, there are significant concerns about the finding. First, a direct pathway from ACC to the hippocampus does not exist in rats or primates. This has been studied with a variety of methods by several leading investigators. In view of the newness of the report in mice, the pathway has not yet been validated using different methods—a step that is critical given the significant discrepancy with other investigations. In addition, even if results are confirmed after appropriate controls, it is unclear how the finding in mice translates to the human in view of its absence in non-human primates.
References
- Andersen P, Holmqvist B, Voorhoeve PE. Entorhinal activation of dentate granule cells. Acta Physiologica Scandinavica. 1966;66:448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nat. 2001;410(6826):131–134. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Hanslmayr S. Neural mechanisms of motivated forgetting. Trends Cogn Sci. 2014;18:279–292. doi: 10.1016/j.tics.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Huddleston E. Towards a Cognitive and Neurobiological Model of Motivated Forgetting. In: Belli RF, editor. True and false recovered memories: Toward a reconciliation of the debate. Vol. 58: Nebraska Symposium on Motivation. New York: Springer; 2011. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner K, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover G, Gabrieli JDE. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute J, Pinto A, Pare D. Ultrastructural organization of medial prefrontal inputs to the rhinal cortices. Eur J Neurosci. 2006;24:135–144. doi: 10.1111/j.1460-9568.2006.04894.x. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends in Cogn Sci. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Barbas H. General Cortical and special Prefrontal Connections: Principles from Structure to Function. Annu Rev Neurosci. 2015;38:269–289. doi: 10.1146/annurev-neuro-071714-033936. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Barbas H, Bunce JG, Medalla M. Prefrontal pathways that control attention. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Functions. Oxford University Press; 2013. pp. 31–48. [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1991;313:65–94. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol. 1981;200:407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM. Cortical afferent input to the principalis region of the rhesus monkey. Neurosci. 1985;15:619–637. doi: 10.1016/0306-4522(85)90064-8. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex. 1997;7:635–646. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. Neuroscientist. 2007;13:532–545. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T, Döhring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Nat Acad Sciences. 2011;108(42):17562–17567. doi: 10.1073/pnas.1110266108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell SA, Billett EE, Crofts JJ, MacDonald DM, Tinsley CJ. The topology of connections between rat prefrontal and temporal cortices. Front Syst Neurosci. 2015;9:80. doi: 10.3389/fnsys.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit R, Anderson MC. Opposing mechanisms support the voluntary forgetting of unwanted memories. Neuron. 2012;76:450–460. doi: 10.1016/j.neuron.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Hulbert JC, Huddleston E, Anderson MC. Adaptive top–down suppression of hippocampal activity and the purging of intrusive memories from consciousness. J Cogn Neurosci. 2014 doi: 10.1162/jocn_a_00696. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neurosci. 1999;92:15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- Biella G, Uva L, de Curtis M. Propagation of neuronal activity along the neocortical-perirhinal-entorhinal pathway in the guinea pig. J Neurosci. 2002;22:9972–9979. doi: 10.1523/JNEUROSCI.22-22-09972.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ, Pandya DN, Rosene DL. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: an anatomical and neurophysiological study. J Comp Neurol. 2003;466:161–179. doi: 10.1002/cne.10866. [DOI] [PubMed] [Google Scholar]
- Bokor H, Csaki A, Kocsis K, Kiss J. Cellular architecture of the nucleus reuniens thalami and its putative aspartatergic/glutamatergic projection to the hippocampus and medial septum in the rat. Eur J Neurosci. 2002;16:1227–1239. doi: 10.1046/j.1460-9568.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Alonso A, Canfield DR, Amaral DG. Dendritic morphology, local circuitry, and intrinsic electrophysiology of principal neurons in the entorhinal cortex of macaque monkeys. J Comp Neurol. 2004;470:317–329. doi: 10.1002/cne.20014. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Barbas H. Prefrontal pathways target excitatory and inhibitory systems in memory-related medial temporal cortices. Neuroimage. 2011;55:1461–1474. doi: 10.1016/j.neuroimage.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JG, Zikopoulos B, Feinberg M, Barbas H. Parallel prefrontal pathways reach distinct excitatory and inhibitory systems in memory-related rhinal cortices. J Comp Neurol. 2013;512:4260–4283. doi: 10.1002/cne.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O’Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14(2):439–453. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Pereira de Vasconcelos A. Importance of the ventral midline thalamus in driving hippocampal functions. Prog Brain Res. 2015;219:145–161. doi: 10.1016/bs.pbr.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and beh. implications. Prog in Neurobiol. 2013;111:34–52. doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino A, Küpper CS, Werner-Seidler A, Dalgleish T, Anderson MC. Failing to Forget Inhibitory-Control Deficits Compromise Memory Suppression in Posttraumatic Stress Disorder. Psychol science. 2015 doi: 10.1177/0956797615569889. 0956797615569889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci & Biobeh Reviews. 2009;33(5):631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Chamberland S, Topolnik L. Inhibitory control of hippocampal inhibitory neurons. Front Neurosci. 2012;6:165. doi: 10.3389/fnins.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Anderson JR. The ghosts of brain states past: remembering reactivates the brain regions engaged during encoding. Psychol Bull. 2010;136(1):87. doi: 10.1037/a0017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Pare D. The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol. 2004;74:101–110. doi: 10.1016/j.pneurobio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Gonzalez-Albo MC, del Rio MR, Elston GN. Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of the occipital and temporal lobes of the macaque monkey. J Comp Neurol. 1999;412:515–526. doi: 10.1002/(sici)1096-9861(19990927)412:3<515::aid-cne10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG. Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc of the Natl Acad of Sciences of the United States of America. 1989;86:2093–2097. doi: 10.1073/pnas.86.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatour B, Witter MP. Projections from the parahippocampal region to the prefrontal cortex in the rat: evidence of multiple pathways. Eur J Neurosci. 2002;15:1400–1407. doi: 10.1046/j.1460-9568.2002.01973.x. [DOI] [PubMed] [Google Scholar]
- Depue Brendan E, Curran Tim, Banich Marie T. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317(5835):215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Depue BE, Orr JM, Smolker HR, Naaz F, Banich MT. The Organization of Right Prefrontal Networks Reveals Common Mechanisms of Inhibitory Regulation Across Cognitive, Emotional, and Motor Processes. Cereb Cortex. 2015 doi: 10.1093/cercor/bhu324. bhu324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Willcutt EG, Ruzic L, Banich MT. Inhibitory control of memory retrieval and motor processing associated with the right lateral prefrontal cortex: evidence from deficits in individuals with ADHD. Neuropsychologia. 2010;48(13):3909–3917. doi: 10.1016/j.neuropsychologia.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE. A neuroanatomical model of prefrontal inhibitory modulation of memory retrieval. Neurosci & Biobehav Rev. 2012;36(5):1382–1399. doi: 10.1016/j.neubiorev.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermon CR, Barbas H. Contralateral thalamic projections predominantly reach transitional cortices in the rhesus monkey. J Comp Neurol. 1994;344:508–531. doi: 10.1002/cne.903440403. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18(1):193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus Reuniens Thalami Modulates Activity in Hippocampal Field CA1 through Excitatory and Inhibitory Mechanisms. J Neurosci. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Witter MP. Nucleus reuniens thalami innervates gamma aminobutyric acid positive cells in hippocampal field CA1 of the rat. Neurosci Lett. 2000;278:145–148. doi: 10.1016/s0304-3940(99)00935-0. [DOI] [PubMed] [Google Scholar]
- Duan AR, Varela C, Zhang Y, Shen Y, Xiong L, Wilson MA, Lisman J. Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: relevance to schizophrenia. Biol Psychiatry. 2015;77:1098–1107. doi: 10.1016/j.biopsych.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Ann Rev of neurosci. 2007;30:123. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn & Mem. 2007;14(10):645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JM, Benoit RG, Gagnepain P, Salman A, Bartholdy S, Bradley C, Chan DKY, Roche A, Brewin CR, Anderson MC. The origins of repetitive thought in rumination: Separating cognitive style from deficits in inhibitory control over memory. J Behav Ther and Exp Psychiatry. 2015;47:1–8. doi: 10.1016/j.jbtep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Funahashi S. Prefrontal cortex and working memory processes. Neurosci. 2006;139:251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 4. Elsevier/Academic Press; 2008. [Google Scholar]
- Gagnepain P, Henson RN, Anderson MC. Suppressing unwanted memories reduces their unconscious influence via targeted cortical inhibition. Proc of the Natl Acad of Sciences. 2014;111(13):E1310–E1319. doi: 10.1073/pnas.1311468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germuska M, Saha S, Fiala JC, Barbas H. Synaptic distinction of laminar specific prefrontal-temporal pathways in primates. Cereb Cortex. 2006;16:865–875. doi: 10.1093/cercor/bhj030. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotions: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neurosci. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Connectivity of GABAergic calretinin-immunoreactive neurons in rat primary visual cortex. Cereb Cortex. 1999;9:683–696. doi: 10.1093/cercor/9.7.683. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Kramar C, Garagoli F, Rossato JI, Weisstaub N, Cammarota M, Medina JH. Medial prefrontal cortex is a crucial node of a rapid learning system that retrieves recent and remote memories. Neurobiol Learn Mem. 2013;103:19–25. doi: 10.1016/j.nlm.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Rissman J, Kiani R, Wagner AD. Cortical reinstatement mediates the relationship between content-specific encoding activity and subsequent recollection decisions. Cerebral Cortex. 2014;24(12):3350–3364. doi: 10.1093/cercor/bht194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL. Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Front Syst Neurosci. 2015;9:29. doi: 10.3389/fnsys.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SHC, Jones EG, Emson PC, Lawson DEM, Heizmann CW, Streit P. Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res. 1989;76:467–472. doi: 10.1007/BF00247904. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hertel PT, Large D, Stück ED, Levy A. Suppression-induced forgetting on a free-association test. Memory. 2012;20(2):100–109. doi: 10.1080/09658211.2011.647036. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, McClure SM. Hierarchical control over effortful behavior by rodent medial frontal cortex: A computational model. Psychol Rev. 2015;122:54–83. doi: 10.1037/a0038339. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. Entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. J Comp Neurol. 2008;509:608–641. doi: 10.1002/cne.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Insausti R, Munoz M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis) Euro J Neurosci. 2001;14:435–451. doi: 10.1046/j.0953-816x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nat. 2015;522:50–55. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- Joormann J, Hertel PT, LeMoult J, Gotlib IH. Training forgetting of negative material in depression. J Abnorm Psychol. 2009;118(1):34. doi: 10.1037/a0013794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara R, Takashima I, Mimura Y, Witter MP, Iijima T. Amygdala input promotes spread of excitatory neural activity from perirhinal cortex to the entorhinal-hippocampal circuit. J of Neurophysiol. 2003;89:2176–2184. doi: 10.1152/jn.01033.2002. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci & Biobehav Revs. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kim H. Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: the HERNET model. Hippocampus. 2015;25(4):500–510. doi: 10.1002/hipo.22387. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17:697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, van Haeften T, Witter MP, Lopes da Silva FH. Electrophysiological characterization of interlaminar entorhinal connections: an essential link for re-entrance in the hippocampal-entorhinal system. Euro J Neurosci. 2003;18:3037–3052. doi: 10.1111/j.1460-9568.2003.03046.x. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Witter MP, Van Haeften T. Topographical and laminar organization of subicular projections to the parahippocampal region of the rat. J Comp Neurol. 2003;455:156–171. doi: 10.1002/cne.10472. [DOI] [PubMed] [Google Scholar]
- Koganezawa N, Taguchi A, Tominaga T, Ohara S, Tsutsui K, Witter MP, Iijima T. Significance of the deep layers of entorhinal cortex for transfer of both perirhinal and amygdala inputs to the hippocampus. Neurosci Res. 2008;61:172–181. doi: 10.1016/j.neures.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Kondo H, Witter MP. Topographic organization of orbitofrontal projections to the parahippocampal region in rats. J Comp Neurol. 2014;522:772–793. doi: 10.1002/cne.23442. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat Neurosci. 2007;10(7):908–914. doi: 10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Küpper CS, Benoit RG, Dalgleish T, Anderson MC. Direct suppression as a mechanism for controlling unpleasant memories in daily life. J Exp Psych: General. 2014;143(4):1443. doi: 10.1037/a0036518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: projections to the neocortex. J Comp Neurol. 2002;447:394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Intrinsic projections and interconnections. J Comp Neurol. 2004;472:371–394. doi: 10.1002/cne.20079. [DOI] [PubMed] [Google Scholar]
- Lehn H, Steffenach HA, van Strien NM, Veltman DJ, Witter MP, Håberg AK. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29(11):3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8(4):313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Purging of memories from conscious awareness tracked in the human brain. J Neurosci. 2012;32:16785–16794. doi: 10.1523/JNEUROSCI.2640-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Acad of Sciences. 2011;1224(1):40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludowig E, Moller J, Bien CG, Munte TF, Elger CE, Rosburg T. Active suppression in the mediotemporal lobe during directed forgetting. Neurobiol Learn Mem. 2010;93:352–361. doi: 10.1016/j.nlm.2009.12.001. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Marzi T, Regina A, Righi S. Emotions shape memory suppression in trait anxiety. Front Psychology. 2013;4 doi: 10.3389/fpsyg.2013.01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci. 2006;23:161–179. doi: 10.1111/j.1460-9568.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. Neuron. 2009;61:609–620. doi: 10.1016/j.neuron.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Anterior cingulate synapses in prefrontal areas 10 and 46 suggest differential influence in cognitive control. J Neurosci. 2010;30:16068–16081. doi: 10.1523/JNEUROSCI.1773-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. The anterior cingulate cortex may enhance inhibition of lateral prefrontal cortex via m2 cholinergic receptors at dual synaptic sites. J Neurosci. 2012;32:15611–15625. doi: 10.1523/JNEUROSCI.2339-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Lera P, Feinberg M, Barbas H. Specificity in inhibitory systems associated with prefrontal pathways to temporal cortex in primates. Cereb Cortex. 2007;17(Suppl 1):i136–i150. doi: 10.1093/cercor/bhm068. [DOI] [PubMed] [Google Scholar]
- Meskenaite V. Calretinin-immunoreactive local circuit neurons in area 17 of the cynomolgus monkey, Macaca fascicularis. J Comp Neurol. 1997;379:113–132. [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Martinez-Marcos A, Pro-Sistiaga P, Blaizot X, Arroyo-Jimenez MM, Marcos P, Artacho-Perula E, Insausti R. Convergence of unimodal and polymodal sensory input to the entorhinal cortex in the fascicularis monkey. Neurosci. 2008;151:255–271. doi: 10.1016/j.neuroscience.2007.09.074. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Pro-Sistiaga P, Arroyo-Jimenez MM, Artacho-Perula E, Insausti AM, Marcos P, Cebada-Sanchez S, Martinez-Ruiz J, Munoz M, Blaizot X, Martinez-Marcos A, Amaral DG, Insausti R. Topographical and laminar distribution of cortical input to the monkey entorhinal cortex. J Anat. 2007;211:250–260. doi: 10.1111/j.1469-7580.2007.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bunble in the rhesus monkey. J Comp Neurol. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Munoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Euro J Neurosci. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, Sauvage MM. Encoding and reactivation patterns predictive of successful memory performance are topographically organized along the longitudinal axis of the hippocampus. Hippocampus. 2015 doi: 10.1002/hipo.22491. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Pare D. Learning-related facilitation of rhinal interactions by medial prefrontal inputs. J Neurosci. 2007;27:6542–6551. doi: 10.1523/JNEUROSCI.1077-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Alonso PM, Bunge SA, Anderson MC, Ghetti S. Strength of coupling within a mnemonic control network differentiates those who can and cannot suppress memory retrieval. J Neurosci. 2013;33(11):5017–5026. doi: 10.1523/JNEUROSCI.3459-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JG, Apergis J, Pare D. Low-probability transmission of neocortical and entorhinal impulses through the perirhinal cortex. J of Neurophysiology. 2004;91:2079–2089. doi: 10.1152/jn.01197.2003. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. The organization of double bouquet cells in monkey striate cortex. J of Neurocytol. 1997;26:779–797. doi: 10.1023/a:1018518515982. [DOI] [PubMed] [Google Scholar]
- Peters GJ, David CN, Marcus MD, Smith DM. The medial prefrontal cortex is critical for memory retrieval and resolving interference. Learn Mem. 2013;20:201–209. doi: 10.1101/lm.029249.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cereb Cortex. 2000;10:851–865. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Cur Opin neurobiol. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: Convergence and segregation of processing streams. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci. 2013;16(8):1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Afferents to the entorhinal cortex of the rat studied by the method of retrograde transport of horseradish peroxidase. Exp Neurol. 1977;57:750–765. doi: 10.1016/0014-4886(77)90106-6. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Making new memories: the role of the hippocampus in new associative learning. Annals of the New York Acad of Sciences. 2007;1097:1–11. doi: 10.1196/annals.1379.007. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kohler C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci. 1986;6:3010–3023. doi: 10.1523/JNEUROSCI.06-10-03010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K. Entorhinal cortex and consolidated memory. Neurosci Res. 2014;84:27–33. doi: 10.1016/j.neures.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Beh Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Jolles J, Witter MP, Uylings HB. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39:1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus. 2003;13:943–952. doi: 10.1002/hipo.10144. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 1975a;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res. 1975b;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N. Cortical afferents to the entorhinal cortex of the rhesus monkey. Science. 1972;175:1471–1473. doi: 10.1126/science.175.4029.1471. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- van Kesteren MT, Fernandez G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci U S A. 2010;107:7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct & Funct. 2014;219:911–929. doi: 10.1007/s00429-013-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neurosci. 2006;142:1–20. [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci and Biobeh Reviews. 2015 doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr opinion neurobiol. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Morris RG. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol. 2010;61:49–79. C41–44. doi: 10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- Wang SH, Tse D, Morris RG. Anterior cingulate cortex in schema assimilation and expression. Learn Mem. 2012;19:315–318. doi: 10.1101/lm.026336.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman BJ, Rockland KS. Divergent cortical connections to entorhinal cortex from area TF in the macaque. J Comp Neurol. 1997;389:361–376. [PubMed] [Google Scholar]
- White EL. Cortical circuits. Synaptic organization of the cerebral cortex Structure, function and theory. Boston: Birkhäuser; 1989. [Google Scholar]
- Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psych Rev. 2013;120(2):329. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, McGarry LM, Vogels TP, Inan M, Anderson SA, Yuste R. State-dependent function of neocortical chandelier cells. J Neurosci. 2011;31:17872–17886. doi: 10.1523/JNEUROSCI.3894-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Jorritsma-Byham B, Goede PH. Combination of anterograde tracing with Phaseolus vulgaris-leucoagglutinin, retrograde fluorescent tracing and fixed-slice intracellular injection of Lucifer Yellow. J Neurosci Methods. 1990;33:207–217. doi: 10.1016/0165-0270(90)90024-a. [DOI] [PubMed] [Google Scholar]
- Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PloS one. 2010;5(11):e13848–e13848. doi: 10.1371/journal.pone.0013848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]