Abstract
A growing body of evidence suggests that nutraceuticals with prolongevity properties may delay the onset of Alzheimer’s disease (AD). We recently demonstrated that a proanthocyanidins-standardized cranberry extract has properties that prolong life span and promote innate immunity in Caenorhabditis elegans. In this article, we report that supplementation of this cranberry extract delayed Aβ toxicity-triggered body paralysis in the C elegans AD model. Genetic analyses indicated that the cranberry-mediated Aβ toxicity alleviation required heat shock transcription factor (HSF)-1 rather than DAF-16 and SKN-1. Moreover, cranberry supplementation increased the transactivity of HSF-1 in an IIS-dependent manner. Further studies found that the cranberry extract relies on HSF-1 to significantly enhance the solubility of proteins in aged worms, implying an improved proteostasis in AD worms. Considering that HSF-1 plays a pivotal role in maintaining proteostasis, our results suggest that cranberry maintains the function of proteostasis through HSF-1, thereby protecting C elegans against Aβ toxicity. Together, our findings elucidated the mechanism whereby cranberry attenuated Aβ toxicity in C elegans and stressed the significance of proteostasis in the prevention of age-related diseases from a practical point of view.
Keywords: Alzheimer’s disease, Cranberry, β-Amyloid, hsf-1, Proteostasis, Caenorhabditis elegans
It has emerged that functional/healthy proteostasis is crucial for organismal health by maintaining the integrity of the proteome (1–3). A healthy proteostasis is maintained by a diverse and complex network that regulates the balance among processes of protein synthesis, folding, and degradation (4,5). Disruption in this balance results in the accumulation of aberrant proteins, which may cause cellular toxicity and lead to numerous age-related disorders (6–8). To date, accumulation, aggregation, and deposition of aberrantly folded proteins have been considered as mechanistic unifying features of many neurodegenerative disorders (9–11). In support of this, diffuse brain atrophy and neuronal lesions are two obvious pathological manifestations of Alzheimer’s disease (AD) (12). Although the detailed molecular mechanisms leading to AD remain unclear, a growing body of evidence implicates that the aggregation of β-amyloid (Aβ) peptide and tau proteins may cause neural loss and cognitive impairment (neuropathological lesions) by forming senile plaques and neurofibrillary tangles outside and inside of neuronal cells, respectively (13,14).
In the United States, AD has been reported as the fifth leading cause of death and the number one cause of institutionalization for people 65 years of age or older (15). Currently about 5.4 million Americans have AD, and the cost of health care to AD patients is approximately up to $200 billion per year. It has been estimated that the number of AD patients will be more than 14 million by 2050, and the health care cost will reach $1.1 trillion (15,16). Thus, AD is becoming a major public health concern for the United States. Contrasting this need, there are only five drugs approved by the Food and Drug Administration (FDA) for the treatment of AD, and these drugs only provide symptomatic benefits for some mild-to-moderate AD patients. Hence, there is a desperate need to find more effective anti-AD agents or means to combat AD, and to that end, it is crucial to have a platform/model to evaluate the effectiveness of certain treatments and reveal their accompanied molecular mechanisms.
Model organisms that mimic human responses offer tremendous opportunities to study the molecular mechanisms of disease and determine the effectiveness of therapeutic strategies. Caenorhabditis elegans has been recently used as model to study the mechanism of β-amyloid (Aβ) toxicity and test the activity of potential anti-AD agents (17–21). Transgenic worms mimicking AD were genetically engineered to express the human Aβ1–42 peptides in either neurons or muscle cells (19,20,22,23). The accumulation of Aβ species in C elegans muscle cells results in either a progressive or rapid paralysis phenotype, providing a simple biological readout of Aβ toxicity (20,23). During the past decade, C elegans AD model has been extensively used to study the protective effects of natural products against AD. Numerous nutraceuticals with prolongevity effects have been shown to prevent or protect against AD (24–29). For instance, in addition to promoting life span and stress resistance, Ginkgo biloba, coffee extract, and soy isoflavone have shown to protect C elegans against Aβ toxicity. The North American cranberry (Vaccinium macrocarpon) and its products have been widely used as nutraceuticals due largely to their anti-microbial, anti-mutagenic, anti-angiogenic, and anti-oxidant properties to human health (30). Recently, we reported that supplementation of a water-soluble cranberry extract standardized to 4.0% PACs (WCESP) can prolong life span of C elegans and Drosophila melanogaster (31,32). Further, health span assays in C elegans indicated that WCESP supplementation also improves animals’ thermotolerance and innate immunity, especially in aged populations (33–35). Our genetic studies revealed that the prolongevity effect of WCESP requires the functional insulin/insulin-like growth factor signaling (IIS) pathway and DAF-16, which have been reported to play a role in protecting C elegans against the toxicity of Aβ aggregation (36). Thus, it is of interest to test whether WCESP has the protective effects in C elegans AD model against Aβ toxicity.
In this study, we engaged CL2006, a transgenic C elegans expressing Aβ in body wall muscle cells, to investigate the beneficial effects of WCESP in AD prevention. WCESP supplementation significantly prolonged the life span of C elegans AD model and also dramatically delayed the progression of body paralysis. These findings indicated that WCESP played a protective role in response to Aβ toxicity. Genetic analyses suggested that this beneficial effect was dependent on the heat shock transcription factor (HSF)-1 rather than daf-16 and skn-1. Our immunoblotting assays found that WCESP supplementation reduced Aβ species in the C elegans AD model. Intriguingly, further studies found that consumption of WCESP increased the solubility of proteins in aged C elegans, implying that WCESP consumption may help maintain a healthy proteostasis in AD worms. The beneficial effects of WCESP on proteostasis maintenance also required HSF-1. Taken together, our findings revealed mechanisms of WCESP’s action against Aβ toxicity and also provide insight into the regulation of healthy aging through dietary intervention.
Experimental Procedures
Strains and Growth Conditions
All strains were maintained at 16 °C on nematode growth medium (NGM) seeded with Escherichia coli OP50 feeding strain. Strains used in this study were as follows: N2 Bristol (wild type), CL2006 (AD worm), and hsf-1 (sy441). All the strains were obtained from the Caenorhabditis Genetics Center (CGC), University of Minnesota.
Preparation of Cranberry Extract
The cranberry extract used in this study was obtained from Naturex-DBS, LLC (Sagamore, MA) and was described previously (33–35). Briefly, the water-soluble fractions of cranberry were spray dried, and the quality and integrity of the WCESP were standardized to 4.0% PACs. A stock solution of WCESP was freshly prepared by dissolving the powder in distilled water to a concentration of 10mg/ml immediately before use, and then the appropriate dilutions were overlaid onto the NGM plates.
RNA Interference
RNA interference (RNAi) clones were grown overnight at 37 °C on Luria Broth plate in the presence of tetracycline (12.5 µg/ml) and carbenicillin (25 µg/ml). Bacterial colonies were inoculated and grown for 8–12 hours, then induced with 2mM isopropyl β-D-1-thiogalactopyranoside for 4 hours at 37 °C. Tenfold concentrated RNAi bacteria were seeded onto RNAi plates containing 25 µg/ml carbenicillin. The RNAi constructs targeting daf-2, age-1, daf-16, hsf-1, and skn-1 were obtained from the C elegans ORFeome RNAi library v1.1.
Life Span Assays
All life span assays were carried out at 20 °C. Synchronous populations were obtained by allowing 10–15 hermaphrodites lay eggs overnight at 16 °C, and the parents were removed the next day. The eggs were allowed to hatch, and 30 L4/young adult worms per plate (NGM plate containing 50µg/ml 5-fluorour-aci1-2′-deoxyribose to prevent the growth of progeny) were used for each assay (37–40). The dead worms were counted starting the next day, and exploding, protruding, bagging, or contaminated worms were censored if applicable. We defined the day when we transferred the L4/young adult worms as Day 0 of adult age. All the assays were carried out in triplicates, and a minimum of three independent trials were performed for all conditions. All statistical analyses were carried out using SPSS software (IBM SPSS Statistics). Kaplan–Meier life span analysis was carried out, and p values were calculated using the log-rank test. p < .05 was accepted as statistically significant.
Worm Paralysis Assays
The assays using strain CL2006 were carried out as described by Cohen and colleagues (36). Synchronous populations of CL2006 worms were prepared on NGM plates by allowing 10–15 hermaphrodites lay eggs overnight at 16 °C, and the parents were removed the next day. The eggs were allowed to hatch, and 20 L4/young adult worms per plate were used for each assay. All paralysis plots were done in triplicates, and a minimum of three independent trials were performed per condition. Nematodes were scored as paralyzed if they exhibited “halos” of cleared bacteria around their heads (indicative of insufficient body movement to access food) or failed to undergo a full body wave propagation upon the nose prodding. Worms were checked every day until all worms were paralyzed. The data were pooled, and the percentage of paralyzed worms was calculated and analyzed using Student’s t-test. p < .05 was accepted as statistically significant.
Pharyngeal Pumping Rate Assay
Pharyngeal pumping rates (the number of contractions of the pharynx terminal bulb in 1 minute) were assayed as previously described with modifications (41). Six to ten worms from each group were transferred to a 60-mm NGM plate seeded with OP50, and the pumping rate was recorded for 15 minutes. Each assay was performed with at least six worms and was repeated at least twice.
Western Blotting of Aβ Species
Rabbit polyclonal Aβ1–42 primary antibodies were from abcam (ab39377). For Western blot analysis, CL2006 worms were synchronized by allowing 10–15 hermaphrodites to lay eggs overnight on WCESP (2mg/ml) containing NGM plates at 16 °C. The parents were removed, and eggs were allowed to hatch and develop to the L4 stage. Subsequently, worms were transferred to WCESP containing NGM plates and continued to grow at 20 °C. CL2006 worms on regular NGM plates without WCESP served as control. After 10 days of growth, worms were transferred to microcentrifuge tubes and washed with S-basal followed by protein immobilization on polyvinylidenefluoride (Bio-Rad) membrane. Polyvinylidenefluoride membrane was incubated with primary antibodies (1:1,000) diluted in 5% nonfat dry milk and then with secondary, HRP-conjugated goat anti-rabbit antibodies (Genscript, A00098; diluted 1:10,000). ACTIN was used as loading control, and the anti-ACTIN antibodies (MAB1501) were from EMD Millipore. Detection was undertaken with standard ECL protocol. Mean intensity of Aβ signals was analyzed using Image-J software (National Institute of Health).
Soluble Protein Extraction
The soluble protein extraction was performed as described previously with alterations (42). Synchronous populations of eggs were prepared by 20% alkaline hypochlorite treatment of gravid adults grown at 16 °C. Eggs were allowed to hatch and develop by transferring to OP50-seeded NGM plates containing 2mg/ml of WCESP at 20 °C. Eggs hatched and developed on OP50-seeded NGM plates without WCESP served as controls. Both worms treated with or without WCESP were collected 10 days after L4 stage. Three separate replicates of each sample (about 200mg [wet weight] of worms) were collected. Total protein extracts were produced in phosphate-buffered saline by sonication on ice, and then the total protein concentration was determined by conducting a bicinchoninic acid assay. Next, the normalized protein samples were spun for 10 minutes at 14,000g to remove the insoluble fraction. The same volume of supernatants (soluble fraction) was loaded and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis . Mean intensities of protein bands were analyzed using Image-J software (National Institute of Health).
Gene Expression Analysis by Quantitative Real-time PCR
CL2006 worms were synchronized by allowing 10–15 hermaphrodites lay eggs overnight on NGM plates containing WCESP at 16 °C. CL2006 worms on regular NGM plates without WCESP served as controls. Worms at the young adult stage were collected with M9 buffer into a ~50–100-µl pellet. RNA was prepared using RNAzol RT reagent (Molecular Research Center) and stored at −80 °C. Complementary DNA was prepared by using Invitrogen Superscript first-strand synthesis system for RT-PCR (Invitrogen). Real-time PCR was performed using using SsoFast EvaGreen Supermix (Bio-Rad) and the CFX96 real-time PCR detection system according to the manufacturer suggested protocol (Bio-Rad). The quantitative real-time PCR (qRT-PCR) conditions were as follows: 95 °C for 3 minutes, followed by 40 cycles of 10 seconds at 95 °C, and 30 seconds at 60 °C. act-1 was used as an internal control to normalize the expression level of target transcripts. Relative fold-changes for transcripts were calculated using the comparative C T (2−ΔΔCT) method (43). Each qRT-PCR experiment was repeated three times using independent RNA/cDNA preparations. The data were pooled and analyzed using student’s t-test, and a p value < .05 was accepted as statistically significant. The qRT-PCR primers for hsf-1 are as follows: 5′-TTGACGACGACAAGCTTCCAGT-3′ (F) and 5′-AAAGCTTGCACCAGAATCATCCC-3′ (R). Primers for hsp-12.6 are as follows: 5′-ATGATGAGCGTTCCAGTGATGGCTGACG-3′ (F) and 5′-TTAATGCATTTTTCTTGCTTCAATGTGAAGAATTCC-3′ (R). Primers for hsp-16.2 are as follows: 5′-TTGCCATCAATCT CAACGTC-3′ (F) and 5′-CTTTCTTTGGCGCTTCAATC-3′ (R). Primers for hsp-70 are as follows: 5′-CGTTTCGAAGAACTGT GTGCTGATCTATTCCGG-3′ (F) and 5′-TTAATCAACTTCCTCA ACAGTAGGTCCTTGTGG-3′ (R). Primers for act-1 are as follows: 5′- CCAGGAATTGCTGATCGTATGCAGAA-3′ (F) and 5′-TGGAGAGGGAAGCGAGGATAGA-3′ (R).
Results
WCESP Supplementation Extends the Life Span of C elegans Model of AD
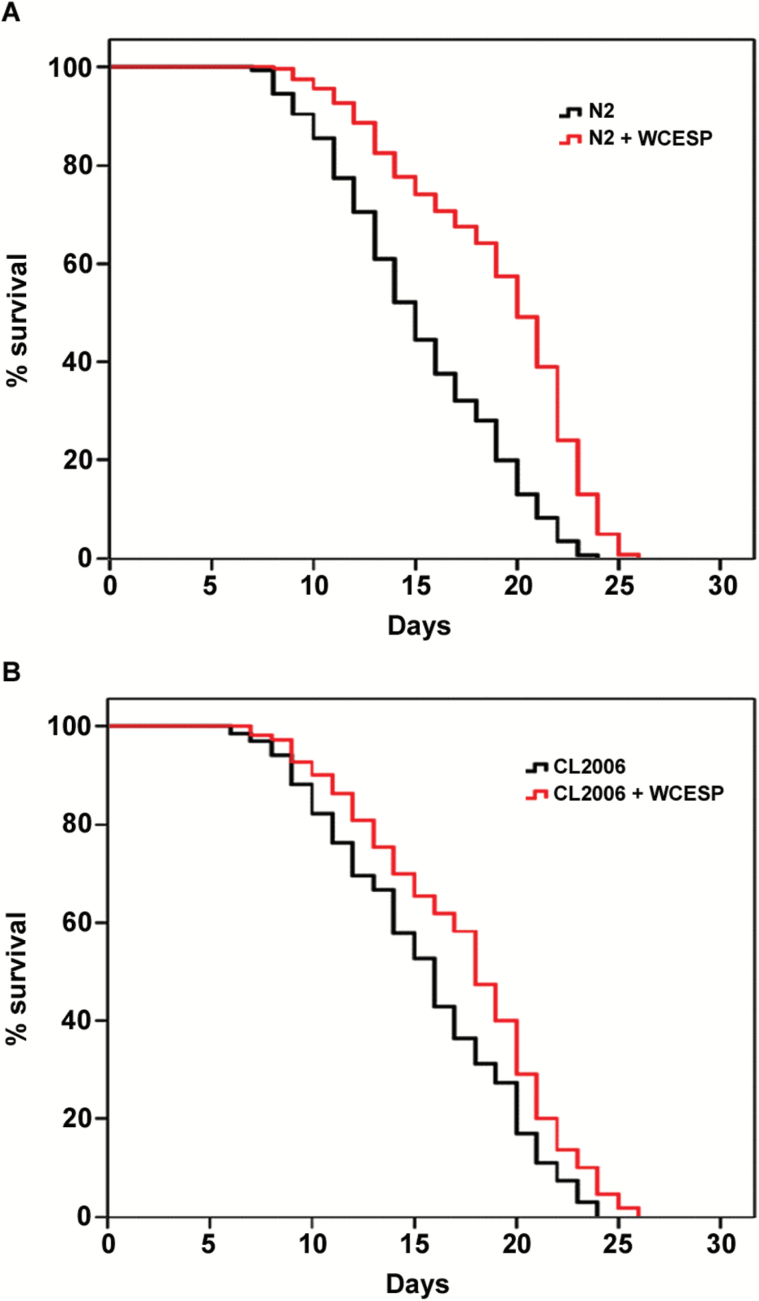
To determine whether the prolongevity effect of cranberry may also benefit the transgenic AD worms, we provided CL2006 worms with or without WCESP and measured their life span at 20 °C. Because it has been reported that 2mg/ml of WCESP is able to significantly prolong C elegans life span (32), we used the same concentration in this study to carry out life span assays in CL2006 AD worms. Our results demonstrated that, consistent with our previous study in wild-type N2 worms (Figure 1A), 2mg/ml of WCESP also significantly extended the life span of CL2006 AD worms (Figure 1B). Specifically, WCESP supplementation extended the mean life span of AD worms from 14.8 to 17.2 days, resulting in a 16.2% increase relative to controls (Figure 1B). Considering the potential link between prolongevity and anti-AD, this finding not only revealed the prolongevity effect of WCESP in the transgenic AD worms but also raised the exciting possibility that WCESP may be effective to AD treatment.
Figure 1.
Water-soluble cranberry extract standardized to 4.0% PAC (WCESP) supplementation prolongs the life span of transgenic C elegans constitutively expressing Aβ. (A) Wild-type N2 worms were treated with 2mg/ml of WCESP. The mean life span of worms (78 N2) treated with WCESP is 19.0±0.29 days, which is significantly longer (p ˂ .001) as compared with that of controls (84 N2, 15.2±0.35 days) without treatment. (B) CL2006, a transgenic C elegans expressing Aβ peptides, was treated with 2mg/ml of WCESP. The mean life span of worms (72 CL2006) treated with WCESP is 17.2±0.58 days, which is significant longer (p = .006) as compared with that of controls (68 CL2006, 14.8±0.59 days) without treatment. Each life span experiment was repeated at least three independent times and similar results were obtained. The life span data were analyzed using SPSS software (IBM SPSS Statistics). Kaplan–Meier life span analysis was carried out, and p values were calculated using the log-rank test.
WCESP Supplementation Delays the Progression of Paralysis in AD Worms
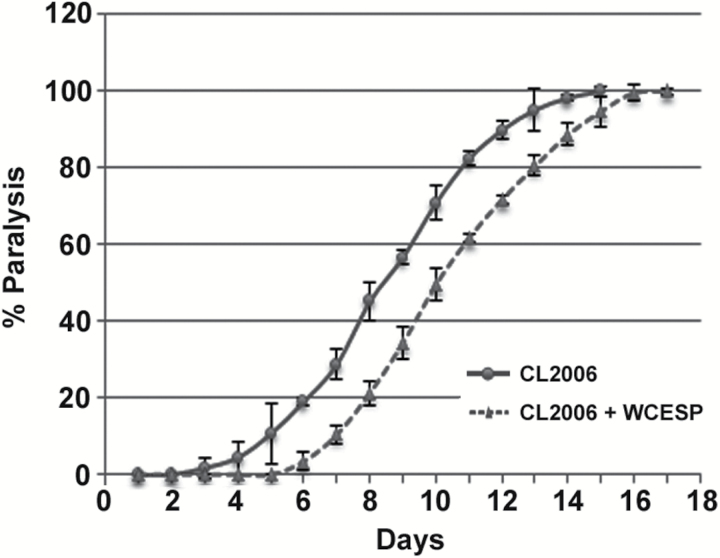
Given the potential beneficial effects of WCESP for treating AD, we examined whether consumption of WCESP in AD worms may interfere with the progression of paralysis induced by Aβ toxicity. Briefly, CL2006 AD worms were treated with or without WCESP (2mg/ml) starting from early L1 stage, and then paralysis assays were conducted when the synchronized CL2006 worms reached the L4/young adult stage. CL2006 worms were checked for paralyses every day until all worms were paralyzed. As compared with controls without WCESP treatment, C elegans CL2006 treated with WCESP showed a dramatic delay of paralysis during the aging process (Figure 2). Considering that the paralysis of CL2006 is driven by Aβ toxicity, our findings suggest that WCESP supplementation might mitigate AD symptoms by alleviating the Aβ toxicity in worms.
Figure 2.
Water-soluble cranberry extract standardized to 4.0% PAC (WCESP) supplementation alleviates Aβ toxicity in C elegans. The WCESP-treated CL2006 worms (dashed line) showed delayed progression of body paralysis as compared with control worms (solid line). Each paralysis assay was conducted in triplicates and repeated at least three times and similar results were obtained. “% paralysis” indicates the average paralysis among the multireplicates and error bars represent the standard deviation.
WCESP Supplementation Decreases Aβ Species in AD Worms
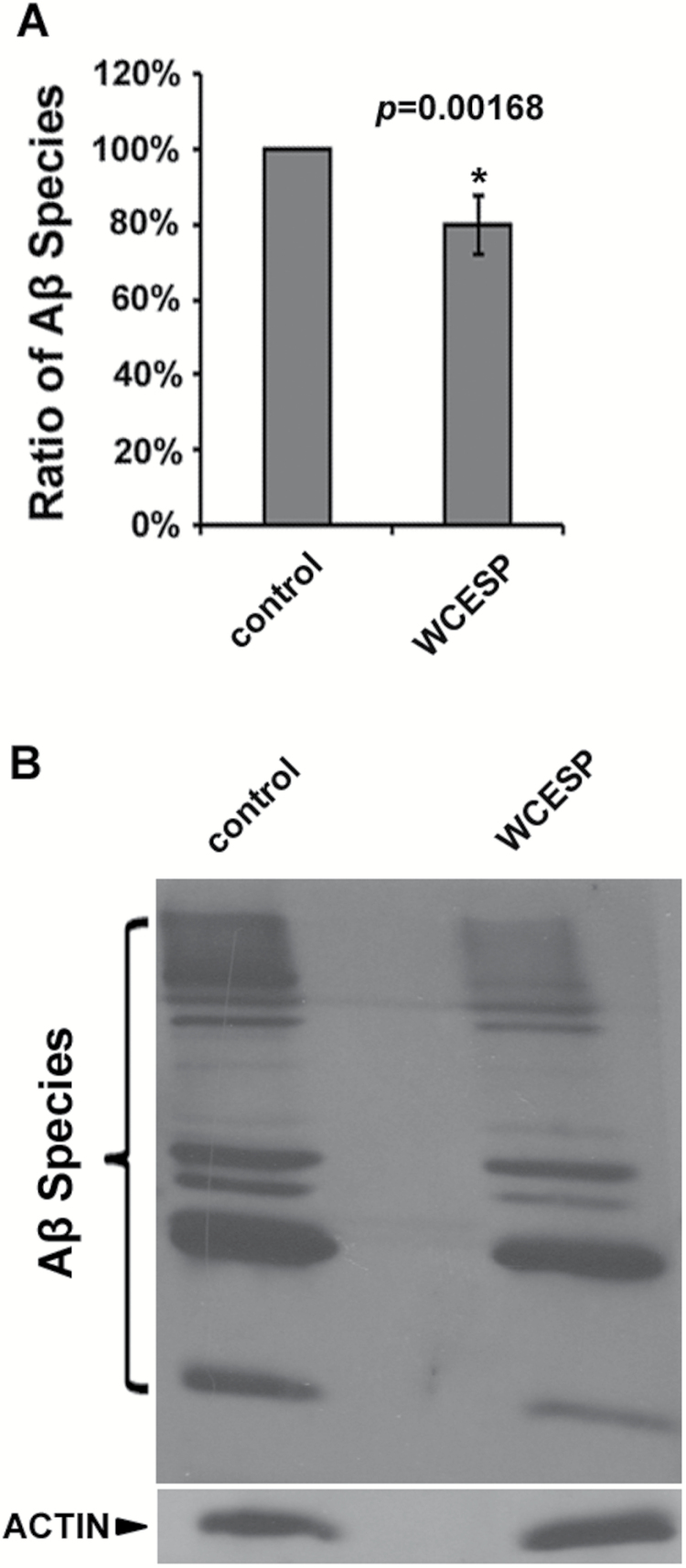
As many botanicals express their protective effects against Aβ toxicity by reducing Aβ species (24,26,44), we were wondering whether WCESP may have the similar effect in AD worms to alleviate Aβ toxicity. To this end, we carried out Western blot analyses to measure the amount of Aβ species in WCESP-treated CL2006 worms by using Image-J software. Our results showed that the total amount of Aβ species in WCESP supplemented AD worms was around 20% less than that in the control AD worms, which was statistically significant (p ˂ .05; Figure 3A). Given these results, we utilized N2 worms to perform similar Western blots and did not observe any reaction band. This suggests that multiple bands observed in AD worms are not necessarily background noise from western blot analysis.
Figure 3.
Water-soluble cranberry extract standardized to 4.0% PAC (WCESP) supplementation reduces the amount of total Aβ species. (A) A significant reduction of intensity is observed in the samples from worms that had been treated with WCESP. Aβ species are quantified by using Image-J software. The graph shows the mean intensity of Aβ species and is the result of three independent experiments. p value was calculated using Student’s t-test. *p < .05 when compared with controls. (B) Immunoblot assay of Aβ species. Western blot analysis was conducted using 10-day-old CL2006 worms.
Intriguingly, it was noticed that the Western signal of each band of Aβ species, from low molecular weight (LMW) Aβ/ Aβ oligomers to high molecular weight (HMW) Aβ aggregates, did not show remarkable reduction in CL2006 worms treated with 2mg/ml of WCESP as compared with controls without WCESP treatment (Figure 3B). It is conceivable that the reduction of overall Aβ species in WCESP-treated AD worms was the accumulation of negligible reduction of each Aβ species. These findings suggest that WCESP supplementation decreases the level of total Aβ species and consequently attenuates Aβ toxicity in AD worms.
WCESP Requires hsf-1, Rather Than daf-16 and skn-1, to Protect AD Worms Against Aβ Toxicity
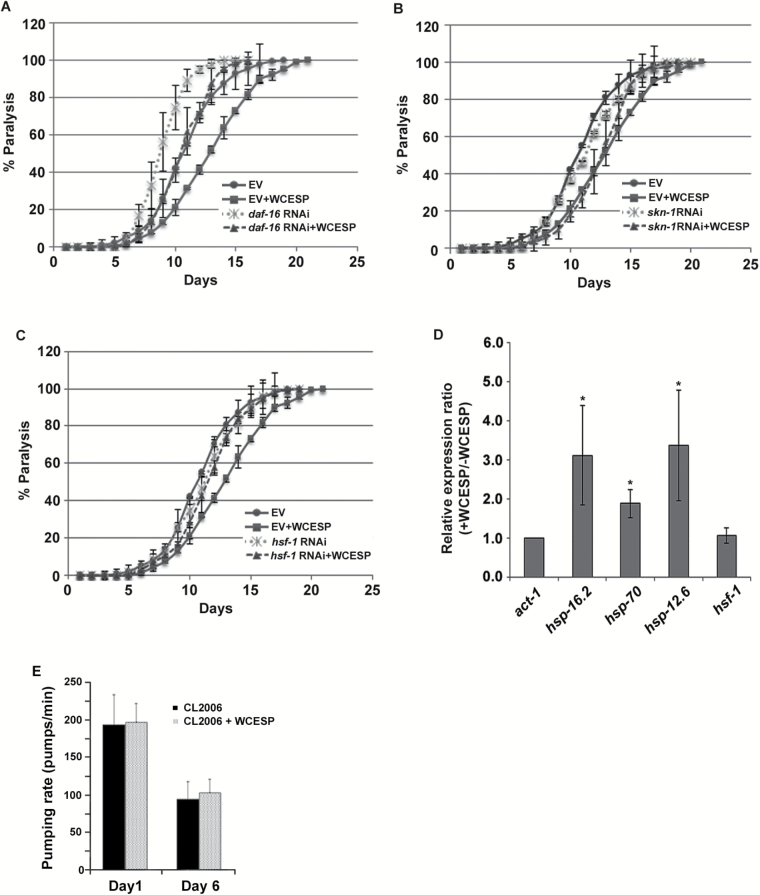
It has been reported that hsf-1, daf-16, and skn-1 play important roles in regulating Aβ aggregation and thereby protecting C elegans from Aβ toxicity (9,26,36). Thus we wondered whether WCESP requires hsf-1, daf-16, or skn-1 to protect against Aβ toxicity in C elegans. To address this concern, genetic epistasis assays were performed in CL2006 worms supplemented with WCESP. In brief, the paralysis progression of CL2006 worms was monitored when RNA interference (RNAi) was applied to individually knock down the gene expression of hsf-1, daf-16, or skn-1. CL2006 transgenic worms were synchronized by laying eggs at 16 °C on NGM plates containing WCESP seeded with particular RNAi or a paired control (empty vector, EV) bacteria. When worms developed into L4/young adult, paralysis assays were carried out every day until all worms were paralyzed. We first examined whether daf-16 RNAi or skn-1 RNAi withdraws the protective effects of WCESP in delaying the progression of body paralysis in AD worms. As compared with controls, WCESP supplementation delayed the paralysis progression of CL2006 worms fed with either daf-16 RNAi or skn-1 RNAi bacteria. These results indicate that neither daf-16 nor skn-1 is essential in WCESP-mediated protection against Aβ toxicity (Figure 4A and B). Using the same strategy, we next examined hsf-1. Excitingly, the protective effect of WCESP on delaying the paralysis progression was completely abolished in CL2006 worms fed with hsf-1 RNAi bacteria as compared with controls (Figure 4C). This demonstrates the necessity of hsf-1 in the WCESP-mediated protection against Aβ toxicity. Collectively, our findings suggest that WCESP requires hsf-1 to protect against Aβ toxicity in C elegans, whereas daf-16 and skn-1 may be dispensable.
Figure 4.
Water-soluble cranberry extract standardized to 4.0% PAC (WCESP) requires hsf-1, but not daf-16 and skn-1, to protect against Aβ toxicity. (A) WCESP supplementation can still delay the progression of Aβ toxicity-induced paralysis in Alzheimer’s disease (AD) worms with DAF-16 level reduced by daf-16 RNAi. (B) WCESP supplementation can still delay the progression of body paralysis in AD worms with SKN-1 level reduced by skn-1 RNAi. (C) Reducing HSF-1 by hsf-1 RNAi significantly abolished the beneficial effects of WCESP delaying the progression of body paralysis in AD worms. Each paralysis assay was repeated at least three independent times with similar results. “% paralysis” indicates the average paralysis among the multireplicates and error bars represent the standard deviation. “EV” stands for empty vector. (D) The transcript levels of hsf-1, hsp-12.6, hsp-16.2, and hsp-70 in AD worms treated with and without 2mg/ml WCESP were quantified using qRT-PCR. The data from three independent experiments were pooled to calculate the mean RNA level normalized to the internal control act-1. The standard errors of the mean (SEM) are shown. *p < .05 when compared with nontreated control. (E) Effect of WCESP on pumping rate in CL2006 worms.
Previous studies have reported that WCESP promotes innate immunity in C elegans by modulating the transactivity of HSF-1 (33). Given that hsf-1 is required in WCESP-mediated protection against Aβ toxicity, we hypothesized that WCESP may also promote the transactivity of HSF-1 in AD worms. To test this hypothesis, we performed qRT-PCR in CL2006 worms to measure the gene expression of hsf-1 and its representative target genes hsp-12.6, hsp-16.2, and hsp-70. CL2006 worms fed with hsf-1 RNAi bacteria served as a system control. Our results indicated that the expression of all these heat shock protein (HSP) genes was upregulated in CL2006 worms supplemented with WCESP, whereas the expression level of hsf-1 was not altered (Figure 4D). In contrast, WCESP-mediated upregulation of HSP genes was completely abolished in hsf-1 RNAi–treated CL2006 worms (data not shown). Collectively, our findings suggest that WCESP supplementation may promote the transactivity of HSF-1 probably through posttranslational regulation rather than through overexpression of hsf-1 gene in AD worms.
Intriguingly, Steinkraus and colleagues previously demonstrated that dietary restriction suppressed proteotoxicity by an hsf-1-dependent mechanism in C elegans (45). Thus, we wondered whether WCESP may mimic dietary restriction by either inhibiting the growth of bacteria or reducing pharyngeal pumping rate of AD worms. As our previous study already reported that WCESP supplementation at 2mg/ml did not inhibit the growth of Escherichia coli OP50 at any bacterial growth phases (34), we accordingly measured the pumping rate of CL2006 AD worms at their adulthood Day 1 and Day 6, respectively. Our results showed that WCESP supplementation did not reduce pumping rates of AD worms and even slightly increased the pumping rates as compared with controls without WCESP treatment (Figure 4E). These findings suggested that WCESP protects AD worms against Aβ toxicity not due to reduced food consumption, although we cannot rule out the possibility that WCESP and dietary restriction act through similar downstream mechanisms.
IIS Is Required for WCESP to Protect AD Worms Against Aβ Toxicity
Considering that IIS regulates the transactivity of HSF-1 (46–48), we next examined whether WCESP’s ability to delay the progression of paralysis in CL2006 worms is dependent on IIS. To this end, we examined daf-2 and age-1, two major components of IIS pathway. Specifically, AD worms CL2006 supplemented with WCESP were fed with RNAi bacteria of daf-2 and age-1, respectively, and the paralysis progression of these worms was monitored. CL2006 worms fed with control RNAi (empty vector) bacteria served as controls. As compared with controls, WCESP consumption did not further delay the progression of paralysis in AD worms fed with either daf-2 RNAi bacteria (Figure 5A) or age-1 RNAi bacteria (Figure 5B). These findings suggest that WCESP may act, at least partially, through IIS to protect C elegans against Aβ toxicity. To further confirm that WCESP may act through IIS to regulate HSF-1’s transactivity, we performed qRT-PCR to measure the expression of hsp-16.2, one of the HSF-1 targets, in AD worms treated with daf-2 RNAi and age-1 RNAi. In consistent with our expectation, RNAi of daf-2 and age-1 significantly eliminated the upregulation of hsp-16.2 in WCESP-treated AD worms (Figure 5C). These findings suggested that WCESP acts through IIS to regulate the transactivity of HSF-1.
Figure 5.
Water-soluble cranberry extract standardized to 4.0% PAC (WCESP) acts through insulin/insulin-like growth factor signaling (IIS) to protect against Aβ toxicity in Alzheimer’s disease (AD) worms. (A) WCESP supplementation cannot further delay the progression of Aβ toxicity-induced paralysis in AD worms with reduced IIS by daf-2 RNAi. (B) WCESP supplementation cannot further delay the progression of body paralysis in AD worms with reduced IIS by age-1 RNAi. Each paralysis assay was repeated at least three independent times and similar results were obtained. “% paralysis” indicates the average paralysis among the multireplicates and error bars represent the standard deviation. “EV” stands for empty vector. (C) The mRNA levels of hsp-16.2 in AD worms treated with RNAi of daf-2 and age-1 were quantified using qRT-PCR, when supplemented with or without 2mg/ml WCESP. The data from three independent experiments were pooled to calculate the mean RNA level normalized to the internal control act-1. The standard errors of the mean (SEM) are shown. The normalized mean RNA level of controls is set to “1”.
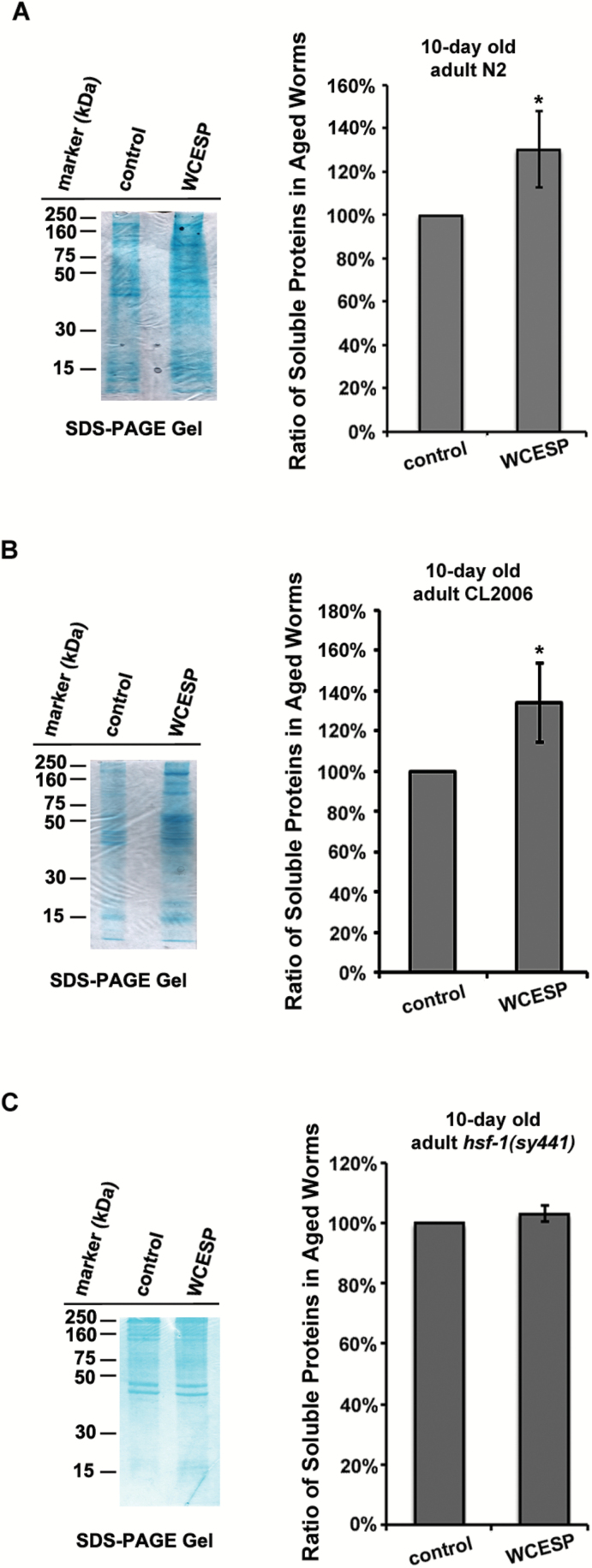
WCESP Supplementation Significantly Increases Solubility of Proteins in Aged C elegans
Aging and age-related disorders are often associated with the deposition of insoluble proteins, indicating that healthy aging requires the proper maintenance of protein conformation (1,42,49). Given that WCESP granted beneficial effects toward both prolongevity and mitigation of body paralysis in AD worms, we next questioned whether WCESP supplementation might lead to improved solubility of proteins in aged C elegans. To address this question, we treated N2 and CL2006 worms with or without WCESP and examined the solubility of proteins in 10-day-old adult worms. Briefly, the synchronous worms treated with or without WCESP were collected 10 days after the L4 stage. Total protein extracts were yielded by sonication, and then the soluble fractions were obtained by removing the insoluble fraction via high-speed centrifugation. Soluble fraction samples were analyzed through a combination of sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Image-J software. Both N2 and CL2006 worms treated with WCESP yielded significantly more soluble proteins relative to the controls without WCESP treatment (Figure 6A and B). Considering that the solubility of cellular proteins plays a vital role in regulating the function of proteostasis (49,50), our results suggest that supplementation with WCESP could improve the function of proteostasis in aged worms.
Figure 6.
Water-soluble cranberry extract standardized to 4.0% PAC (WCESP) improves proteostasis in aged C elegans dependent on HSF-1. (A) WCESP supplementation increases protein solubility in 10-day-old N2 worms. (B) WCESP supplementation increases protein solubility in 10-day-old CL2006 Alzheimer’s disease (AD) worms. (C) WCESP supplementation cannot increase protein solubility in 10-day-old hsf-1 (sy441) mutant worms. Soluble proteins on the sodium dodecyl sulfate–polyacrylamide gel electrophoresis are quantified by using Image-J software. Data are expressed as mean intensity from three independent experiments. The standard errors of the mean (SEM) are shown. p value was calculated using Student’s t-test. *p < .05 when compared with controls.
Given that WCESP requires HSF-1 to promote the healthy aging in C elegans, our finding that WCESP improved the solubility of proteins in aged worms suggests that WCESP might also require HSF-1 to improve protein solubility in C elegans. To test this interesting speculation, we supplemented hsf-1 deletion mutant worms with or without WCESP and collected protein samples using the same strategy in Figure 6A. Intriguingly, hsf-1 deletion mutant worms treated with WCESP yielded similar amount of soluble proteins as were yielded by hsf-1 deletion mutant worms without WCESP treatment (Figure 6C). These findings suggest that WCESP-mediated increase of protein solubility in aged worms is also dependent on HSF-1. Considering that the function of proteostasis in improving the protein solubility in cells, and taking into account the vital role of HSF-1 in regulating proteostasis (4,36), our findings together propose an exciting model of WCESP’s action, that is, WCESP supplementation may modulate the activities of HSF-1 to help maintain or improve the function of proteostasis in C elegans, thereby ameliorating Aβ toxicity and thus delaying the progression of body paralysis in AD worms.
Discussion
Previous in vitro studies proposed that cranberry extract may have some protective effects against AD (51). Thus, it is interesting to further test this beneficial effect in vivo and reveal the underlying molecular mechanisms accordingly. Our recent studies have demonstrated that a unique extract from the North American cranberry (Vaccinium macrocarpon), WCESP, is potent to promote longevity and healthy aging in model organisms (31,33–35,52,53). Here, we employed CL2006, a transgenic C elegans strain constitutively expressing Aβ, as an AD model to investigate the protective effects of WCESP against Aβ toxicity in vivo and elucidated molecular mechanisms underlying this protection. Excitingly, our results indicated that supplementation of WCESP substantially extended life span of the AD worms and delayed the progression of their Aβ toxicity-induced body paralysis. Our epistasis analyses suggested that WCESP requires hsf-1, rather than daf-16 and skn-1, to protect against Aβ toxicity in AD worms. Mechanistic studies further demonstrated that WCESP modulates the transactivity of HSF-1 through IIS cascade. Moreover, WCESP consumption results in the reduction of Aβ species in AD worms and greatly increases the solubility of proteins in aged worms, which is a hallmark of well-maintained proteostasis (5,42). Intriguingly, the improvement of protein solubility conferred by WCESP supplementation in AD worms also required the function of HSF-1. Considering the imperative role of HSF-1 in regulating proteostasis, our findings suggest that WCESP may alleviate Aβ toxicity by improving the function of proteostasis in C elegans, which required HSF-1 in an IIS-dependent manner.
Previous studies reported that DAF-16, SKN-1, and HSF-1 played pivotal roles in regulating longevity and ameliorating Aβ toxicity (36,54,55). Thus we wondered whether these regulators may participate in the WCESP-mediated prevention against Aβ toxicity. To this end, we employed RNAi techniques to knock down the expression of aforementioned gene in CL2006 AD worms and then monitored the progression of their Aβ toxicity-induced body paralysis. Our results showed that RNAi of either daf-16 or skn-1 did not abolish WCESP-mediated progression delay of body paralysis (Figure 4A and B), whereas RNAi of hsf-1 in CL2006 AD worms completely abolished the progression delay of paralysis relative to controls (Figure 4C). These findings indicated that WCESP’s protection against Aβ toxicity in C elegans is mainly dependent on HSF-1. Given that inefficient proteostasis results in the accumulation of aggregates (4), such as Aβ aggregates, we reasoned that WCESP supplementation might protect AD worms against Aβ toxicity by maintaining a functional healthy proteostasis. Considering that HSF-1 plays a pivotal role in regulating proteostasis (36), our results suggested that WCESP supplementation may improve the function of proteostasis by promoting the transactivity of HSF-1 in AD worms. In support of this, our further qRT-PCR experiments measured the expression of several major target genes of HSF-1 and showed that these genes were upregulated in CL2006 AD worms supplemented with WCESP as compared with controls without WCESP treatment (Figure 4D). A growing body of evidence demonstrated that HSF-1 plays imperative roles in regulating protein synthesis, folding, and degradation (1,56), implying that HSF-1 may participate in the whole process of proteostasis regulation. Therefore, our findings indicated that WCESP may influence the entire process of proteostasis through HSF-1.
We previously reported that WCESP acted through IIS cascade to promote activity of HSF-1 in C elegans (33). Given that HSF-1 was required for the WCESP-mediated protective effects against Aβ toxicity, we speculated that WCESP may also act through IIS in the transgenic AD worms to modulate the activity of HSF-1. In support of our speculation, WCESP supplementation did not further delay the progression of body paralysis in AD worms with reduced IIS (Figure 5A and B), suggesting that IIS is indispensable in WCESP-mediated protection against Aβ toxicity. Given that IIS was able to regulate both daf-16 and hsf-1 to protect against Aβ toxicity and delay the progression of paralysis in CL2006 worms (36), our finding that WCESP mainly relied on HSF-1 to protect against Aβ toxicity actually raised a very interesting possibility, that is, some other factors may also be required in concert with IIS to specify the transactivity of HSF-1 in response to WCESP treatment. Apparently, more experiments are needed to thoroughly unravel the sophisticated mechanisms whereby the regulatory specificity of HSF-1 is determined in response to WCESP treatment. Undoubtedly, the results of these experiments will not only unveil the mechanisms at the molecular level, whereby WCESP acts to promote proteostasis and the stability of the proteome, but also provide valuable insight into how HSF-1 is fine-tuned to modulate healthy aging from a fundamental point of view.
Healthy aging is associated with the healthy proteostasis, which is able to maintain the balance among processes of protein synthesis, folding, and degradation. In contrast, imbalance among these processes results in the aggregation and deposition of aberrant proteins, leading to aging and age-related diseases. Of note, Reis-Rodrigues and colleagues showed that the solubility of cellular proteins declined with the aging process, suggesting that better protein solubility is a hallmark of healthy proteostasis (42). Our protein solubility experiments revealed that WCESP supplementation dramatically increased the solubility of proteins in aged worms (Figure 6A and B), suggesting that WCESP possessed the ability to maintain a healthy proteostasis in C elegans. Taking into account that a healthy proteostasis could alleviate Aβ toxicity, our finding that WCESP relied on HSF-1 to delay Aβ toxicity-induced body paralysis of worms indicated that WCESP required HSF-1 to maintain a healthy proteostasis in C elegans. Indeed, we validated this surmise by comparing protein solubility in hsf-1 deletion mutant worms treated with and without WCESP. Our results indicated that the solubility of proteins in hsf-1 deletion mutants was not altered no matter whether WCESP was present or absent (Figure 6C). Collectively, our findings demonstrated that WCESP helps maintain the proteostasis and thereby alleviates Aβ toxicity in CL2006 AD worms in an hsf-1-dependent manner.
Interestingly, Cohen and colleagues previously reported that HSF-1 alleviated Aβ toxicity by first disaggregating large Aβ aggregates into small Aβ aggregates and next promoting the degradation of small Aβ aggregates into peptides or amino acids (36). Considering that aggregation and disaggregation of protein molecules are a reversible process, their fantastic finding actually implied that HSF-1 preferentially drove the disaggregation process toward degradation of small Aβ aggregates in AD worms. Therefore, HSF-1-triggered alleviation of Aβ toxicity should be associated with the reduction of all Aβ species rather than only high molecular weight (HMW) Aβ aggregates or low molecular weight (LMW) Aβ/ Aβ oligomers. Our results showed that WCESP supplementation did not remarkably reduce the amount of particular Aβ species in range from LMW Aβ/ Aβ oligomers to HMW Aβ aggregates, whereas the total amount of Aβ species in WCESP-treated AD worms was significantly reduced as compared with control worms without WCESP treatment (Figure 3A and B). Given that WCESP protected against Aβ toxicity dependent on HSF-1, our results actually further supported the findings that Cohen and colleagues reported from a practical point of view.
Taken together, our findings elucidated some of the molecular mechanisms by which WCESP alleviated Aβ toxicity in C elegans and highlighted the beneficial effects of WCESP on AD prevention. Although health effects of WCESP have been studied for many years, our study is the first to thoroughly investigate the properties of WCESP on protection against Aβ toxicity and the first to systematically analyze the genetic requirements for WCESP-mediated anti-AD effects. Because HSF-1 is highly conserved in species ranging from C elegans to mammals, our finding that HSF-1 is engaged to maintain proteostasis in response to WCESP supplementation has immediate implications for the utilization of WCESP in promoting healthy aging by combating age-related diseases in higher order organisms.
Funding
This study was funded by Intramural Research Program at the National Institute on Aging, NIH (to S.Z.) and China National Science and Technology Major Projects for Key New Drugs Innovation (to B.Y., 2012ZX09103301-018).
Acknowledgments
Caenorhabditis elegans strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). We are grateful to members of the Dong laboratory for helpful discussions. We especially thank E. Wilson and J. Angeloni for editing this article.
References
- 1. Lindquist SL, Kelly JW. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol. 2011;3:a004507. doi:10.1101/cshperspect.a004507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saez I, Vilchez D. The mechanistic links between proteasome activity, aging and age-related diseases. Curr Genomics. 2014;15:38–51. doi:10.2174/ 138920291501140306113344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vilchez D, Saez I, Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun. 2014;5:5659. doi:10.1038/ncomms6659 [DOI] [PubMed] [Google Scholar]
- 4. Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi:10.1126/science.1141448 [DOI] [PubMed] [Google Scholar]
- 5. Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci. 2009;64:167–170. doi:10.1093/gerona/gln071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosco DA, LaVoie MJ, Petsko GA, Ringe D. Proteostasis and movement disorders: Parkinson’s disease and amyotrophic lateral sclerosis. Cold Spring Harb Perspect Biol. 2011;3:a007500. doi:10.1101/cshperspect.a007500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finkbeiner S. Huntington’s disease. Cold Spring Harb Perspect Biol. 2011;3:a007476. doi:10.1101/cshperspect.a007476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selkoe DJ. Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2011;3:a004457. doi:10.1101/cshperspect.a004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dillin A, Cohen E. Ageing and protein aggregation-mediated disorders: from invertebrates to mammals. Philos Trans R Soc Lond B Biol Sci. 2011;366:94–98. doi:10.1098/rstb.2010.0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging. 2000;21:719–727. [DOI] [PubMed] [Google Scholar]
- 11. Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2:E207–E209. doi:10.1038/35041139 [DOI] [PubMed] [Google Scholar]
- 12. Madeo J, Frieri M. Alzheimer’s disease and immunotherapy. Aging Dis. 2013;4:210–220. [PMC free article] [PubMed] [Google Scholar]
- 13. Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masters CL, Selkoe DJ. Biochemistry of amyloid beta-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Biol. 2012;2:a006262. doi:10.1101/cshperspect.a006262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tejada-Vera B. Mortality from Alzheimer’s disease in the United States: data for 2000 and 2010. NCHS data brief. 2013:1–8. [PubMed] [Google Scholar]
- 16. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ewald CY, Li C. Understanding the molecular basis of Alzheimer’s disease using a Caenorhabditis elegans model system. Brain Struct Funct. 2010;214:263–283. doi:10.1007/s00429-009-0235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson JR, Jenn RC, Barclay JW, Burgoyne RD, Morgan A. Caenorhabditis elegans: a useful tool to decipher neurodegenerative pathways. Biochem Soc Trans. 2010;38:559–563. doi:10.1042/BST0380559 [DOI] [PubMed] [Google Scholar]
- 19. Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans . Proc Natl Acad Sci U S A. 1995;92:9368–9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Link CD. C. elegans models of age-associated neurodegenerative diseases: lessons from transgenic worm models of Alzheimer’s disease. Exp Gerontol. 2006;41:1007–1013. doi:10.1016/j.exger.2006.06.059 [DOI] [PubMed] [Google Scholar]
- 21. Wolozin B, Gabel C, Ferree A, Guillily M, Ebata A. Watching worms whither: modeling neurodegeneration in C. elegans . Prog Mol Biol Transl Sci. 2011;100:499–514. doi:10.1016/B978-0-12-384878-9.00015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McColl G, Roberts BR, Gunn AP, et al. The Caenorhabditis elegans A beta 1–42 model of Alzheimer disease predominantly expresses A beta 3–42. J Biol Chem. 2009;284:22697–22702. doi:10.1074/jbc.C109.028514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dostal V, Link CD. Assaying beta-amyloid toxicity using a transgenic C. elegans model. J Vis Exp. 2010;44:2252. doi:10.3791/2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abbas S, Wink M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine. 2010;17:902–909. doi:10.1016/j.phymed.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 25. Arya U, Dwivedi H, Subramaniam JR. Reserpine ameliorates Abeta toxicity in the Alzheimer’s disease model in Caenorhabditis elegans . Exp Gerontol. 2009;44:462–466. doi:10.1016/j.exger.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 26. Dostal V, Roberts CM, Link CD. Genetic mechanisms of coffee extract protection in a Caenorhabditis elegans model of β-amyloid peptide toxicity. Genetics. 2010;186:857–866. doi:10.1534/genetics.110.120436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gutierrez-Zepeda A, Santell R, Wu Z, et al. Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans . BMC Neurosci. 2005;6:54. doi:10.1186/1471-2202-6-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Y, Wu Z, Butko P, et al. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans . J Neurosci. 2006;26:13102–13113. doi:10.1523/JNEUROSCI.3448-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zarse K, Bossecker A, Muller-Kuhrt L, et al. The phytochemical glaucarubinone promotes mitochondrial metabolism, reduces body fat, and extends lifespan of Caenorhabditis elegans . Horm Metab Res. 2011;43:241–243. doi:10.1055/s-0030-1270524 [DOI] [PubMed] [Google Scholar]
- 30. Pappas E, Schaich KM. Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49:741–781. doi:10.1080/10408390802145377 [DOI] [PubMed] [Google Scholar]
- 31. Wang C, Yolitz J, Alberico T, et al. Cranberry interacts with dietary macronutrients to promote healthy aging in drosophila. J Gerontol A Biol Sci Med Sci. 2014;69:945–954. doi:10.1093/gerona/glt161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guha S, Cao M, Kane RM, Savino AM, Zou S, Dong Y. The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age (Dordr). 2013;35:1559–1574. doi:10.1007/s11357-012-9459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dinh J, Angeloni JT, Pederson DB, Wang X, Cao M, Dong Y. Cranberry extract standardized for proanthocyanidins promotes the immune response of Caenorhabditis elegans to Vibrio cholerae through the p38 MAPK pathway and HSF-1. PLoS One. 2014;9:e103290. doi:10.1371/journal.pone.0103290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guha S, Cao M, Kane RM, Savino AM, Zou S, Dong Y. The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age (Dordr). 2013;35:1559–1574. doi:10.1007/s11357-012-9459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guha S, Natarajan O, Murbach CG, et al. Supplement timing of cranberry extract plays a key role in promoting Caenorhabditis elegans healthspan. Nutrients. 2014;6:911–921. doi:10.3390/nu6020911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi:10.1126/science.1124646 [DOI] [PubMed] [Google Scholar]
- 37. Gandhi S, Santelli J, Mitchell DH, Stiles JW, Sanadi DR. A simple method for maintaining large, aging populations of Caenorhabditis elegans . Mech Ageing Dev. 1980;12:137–150. doi:10.1016/0047-6374(80)90090-1 [DOI] [PubMed] [Google Scholar]
- 38. Hosono R. Sterilization and growth inhibition of Caenorhabditis elegans by 5-fluorodeoxyuridine. Exp Gerontol. 1978;13:369–374. [DOI] [PubMed] [Google Scholar]
- 39. Hosono R, Mitsui Y, Sato Y, Aizawa S, Miwa J. Life span of the wild and mutant nematode Caenorhabditis elegans. Effects of sex, sterilization, and temperature. Exp Gerontol. 1982;17:163–172. doi:10.1016/0531-5565(82)90052-3 [DOI] [PubMed] [Google Scholar]
- 40. Mitchell DH, Stiles JW, Santelli J, Sanadi DR. Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J Gerontol. 1979;34:28–36. [DOI] [PubMed] [Google Scholar]
- 41. Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans . Proc Natl Acad Sci U S A. 2004;101:8084–8089. doi:10.1073/pnas.0400848101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reis-Rodrigues P, Czerwieniec G, Peters TW, et al. Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell. 2012;11:120–127. doi:10.1111/j.1474-9726.2011.00765.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 44. Martorell P, Bataller E, Llopis S, et al. A cocoa peptide protects Caenorhabditis elegans from oxidative stress and beta-amyloid peptide toxicity. PloS One. 2013;8:e63283. doi:10.1371/journal.pone.0063283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steinkraus KA, Smith ED, Davis C, et al. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans . Aging Cell. 2008;7:394–404. doi:10.1111/j.1474-9726.2008.00385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148:322–334. doi:10.1016/j.cell.2011.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen E, Du D, Joyce D, et al. Temporal requirements of insulin/IGF-1 signaling for proteotoxicity protection. Aging Cell. 2010;9:126–134. doi:10.1111/j.1474-9726.2009.00541.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Volovik Y, Maman M, Dubnikov T, et al. Temporal requirements of heat shock factor-1 for longevity assurance. Aging Cell. 2012;11:491–499. doi:10.1111/j.1474-9726.2012.00811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A. 2009;106:14914–14919. doi:10.1073/pnas.0902882106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi:10.1101/gad.1657108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Joseph JA, Fisher DR, Carey AN. Fruit extracts antagonize Abeta- or DA-induced deficits in Ca2+ flux in M1-transfected COS-7 cells. J Alzheimers Dis. 2004;6:403–411. [DOI] [PubMed] [Google Scholar]
- 52. Sun Y, Yolitz J, Alberico T, Sun X, Zou S. Lifespan extension by cranberry supplementation partially requires SOD2 and is life stage independent. Exp Gerontol. 2014;50:57–63. doi:10.1016/j.exger.2013.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zou S, Carey JR, Liedo P, Ingram DK, Yu B. Prolongevity effects of a botanical with oregano and cranberry extracts in Mexican fruit flies: examining interactions of diet restriction and age. Age (Dordr). 2012;34:269–279. doi:10.1007/s11357-011-9230-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi:10.1126/science.1083701 [DOI] [PubMed] [Google Scholar]
- 55. Li X, Matilainen O, Jin C, Glover-Cutter KM, Holmberg CI, Blackwell TK. Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet. 2011;7:e1002119. doi:10.1371/journal.pgen.1002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi:10.1038/nature10317 [DOI] [PubMed] [Google Scholar]