Figure 2. Localization, enzymatic activity and modulation of sirtuins by small molecules.
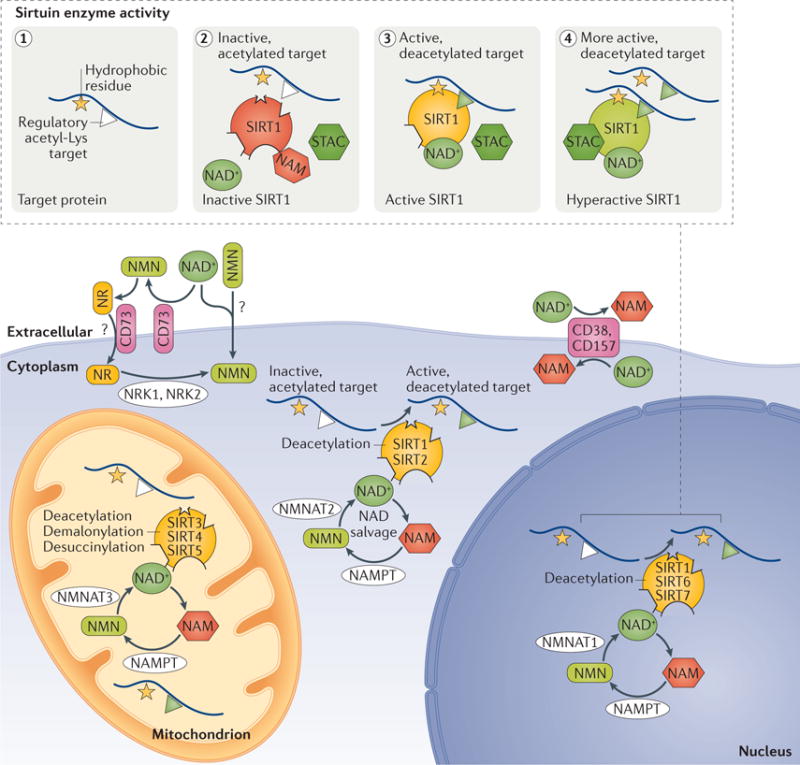
The mammalian sirtuins (SIRT1−7) are a family deacylases that are localized to various cellular compartments. SIRT1, SIRT6 and SIRT7 are localized primarily to the nucleus, whereas SIRT3, SIRT4 and SIRT5 are present in the mitochondria. SIRT1 (in some cell types) and SIRT2 are localized to the cytosol. Their activities are regulated by nicotinamide dinucleotide (NAD+), which is synthesized de novo from Trp, Asp or niacin (not shown). NAD+ is also recycled by the NAD salvage pathway from nicotinamide (NAM) by nicotinamide phosphoribosyltransferase (NAMPT), which converts NAM to nicotinamide mononucleotide (NMN). NMN is converted to NAD+ by nicotinamide mononucleotide adenylyltransferase1 (NMNAT1), NMNAT2 or NMNAT3, which are also capable of converting nicotinic acid mononucleotide (NaMN) to NAD+. Additional NAD+ precursors used in the NAD salvage pathway include nicotinamide riboside (NR) and nicotinic acid riboside, which are converted to NMN and NaMN, respectively (not shown) by nicotinamide riboside kinase 1 (NRK1) and NRK2. The cell membrane-bound glycohydrolases CD38 and CD157 degrade NAD+ to NAM135,142. CD73 converts NAD+ to NMN and NMN to NR; it is a transmembrane protein that may also act as a NR transporter177,178. There is evidence to indicate that NAD+ and NMN can be directly transported across the cell membrane in some cell types, although the mechanism is not yet known. The inset shows that SIRT1 activation is stimulated by the presence of a hydrophobic amino acid adjacently to the target Lys (panel 1). When NAM levels are sufficiently high, it binds into the carboxy-terminal pocket of SIRT1 and inhibits it58 (panel 2). SIRT1 utilizes NAD+ as a co-substrate to promote the binding and deacetylation of specific protein substrates (panel 3). Sirtuin-activating compounds (STACs) activate SIRT1 by binding to the STAC-binding domain and primarily lowering the Km for the peptide substrate, thereby increasing its catalytic activity (panel 4).
