Figure 4. Sirtuin activation and its disease relevance.
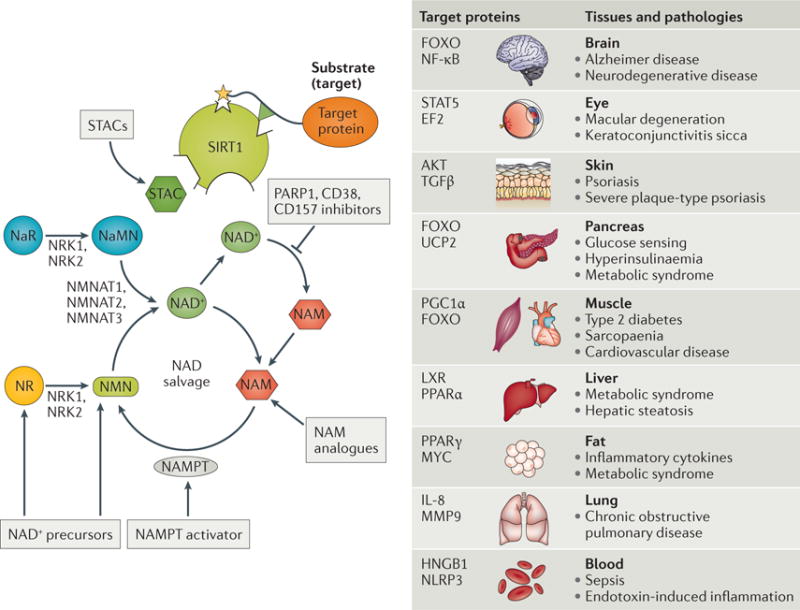
Sirtuin activity can be stimulated through various mechanisms: allosterically by sirtuin-activating compounds (STACs), such as resveratrol, SRT1720 and SRT2104, which lower the Km for the target proteins; increasing nicotinamide adenine dinucleotide (NAD+) levels by providing its precursors nicotinamide riboside (NR) or nicotinamide mononucleotide (NMN); inhibiting the glycohydrolases CD38 or CD157 (with apigenin, quercetin, GSK 897-78c)142,143; inhibiting poly(ADP-ribose) polymerases (PARPs)34; activating nicotinamide phosphoribosyltransferase (NAMPT) with P7C3 (REF. 152); or by providing nicotinamide (NAM) analogues, such as methyl-NAM. Nicotinamide riboside kinase 1 (NRK1) and NRK2 convert NR and nicotinic acid riboside (NaR) is to NMN and nicotinic acid mononucleotide (NaMN), respectively. NaMN and NMN are converted to NAD+ by nicotinamide mononucleotide adenylyltransferase 1 (NMNAT1), NMNAT2 and NMNAT3. Sirtuins directly or indirectly target more than 100 signalling proteins with relevance to human disease in a variety of tissues ranging from brain to blood (reviewed in REF. 41). Although STACs have therapeutic potential, certain diseases, such as cancer in certain contexts and Parkinson disease, may benefit from sirtuin inhibition (reviewed in REF. 179). EF2, elongation factor 2; FOXO, forkhead box O; HNGB1, high-mobility group box 1; IL, interleukin; LXR, liver X receptor; MMP9, matrix metalloproteinase 9; NF-κB, nuclear factor-κB; NLRP3, NOD-, LRR- and pyrin domain-containing 3; PGC1α, peroxisome proliferator-activated receptor-γ co-activator 1α; PPAR, peroxisome proliferator-activated receptor; STAT5, signal transducer and activator of transcription 5; TGFβ, transforming growth factor-β; UCP2, mitochondrial uncoupling protein 2.
