Abstract
Background
Video-assisted thoracic surgery (VATS)-assisted lobectomy is widely used to treat non-small cell lung carcinoma (NSCLC). There are no reports concerning the comparison between single-port VATS and two-port VATS in treating NSCLC. This study aimed to compare the perioperative and short-term follow-up results between these two methods for treating NSCLC.
Methods
A retrospective surgical evaluation of patients undergoing either single-port VATS or two-port VATS for NSCLC between January 2013 and June 2015 was conducted. The propensity score (PS) matching method was used to reduce selection bias by creating two groups. After generating the PSs, 1:1 ratio and nearest-neighbor score matching was completed. The primary outcome measures were surgical time, blood loss, drainage time, length of hospital stay, postoperative pain score and patient satisfaction score. The data were analyzed statistically with P<0.05 defined as statistically significant.
Results
Of the 143 patients who met the inclusion criteria, 66 (46.2%) were operated on using two-port VATS and 77 (53.8%) using single-port VATS. After 1-to-1 PS matching, 63 pairs were selected. Both groups were well balanced for age, gender, body mass index, pulmonary function, preoperative comorbidity, tumor size and tumor type. The single-port VATS group had less blood loss, less postoperative pain, and a higher satisfaction score than those in the two-port VATS group, with statistical significance. Postoperative complications occurred in 2 (2/63, 3.2%) patients in the single-port VATS group and 6 (6/63, 9.5%) patients in the two-port VATS group, not a significant difference. No deaths occurred during the follow-up period.
Conclusions
A single-port VATS-assisted lobectomy is suggested to be safe and feasible for treating NSCLC. Compared with two-port VATS, single-port VATS has many advantages, including reduced blood loss, less postoperative pain and a higher satisfaction score.
Keywords: Lobectomy, non-small cell lung carcinoma (NSCLC), single incision, video-assisted thoracic surgery (VATS)
Introduction
Lung cancer is reported to have the highest fatality rate of pulmonary diseases. Non-small cell lung carcinoma (NSCLC) is any type of epithelial lung cancer other than small cell lung carcinoma and accounts for approximately 85% of all lung cancers (1). NSCLCs are primarily treated by surgical resection, although chemotherapy (i.e., adjuvant chemotherapy) is increasingly being used.
During the past decade, video-assisted thoracic surgery (VATS) has been introduced for the treatment of lung cancer and is thought to be less invasive than a conventional thoracotomy. It has been demonstrated that a VATS lobectomy has several advantages over a thoracotomy, including a shorter hospital stay, shorter duration of chest tube drainage, less postoperative loss of respiratory function, and fewer surgical complications (2-4). In 2010, Gonzalez-Rivas et al. first reported the single port VATS-assisted lobectomy (5). Currently, the single port VATS-assisted lobectomy is used in complex chest surgeries such as lung resection, bronchial sleeve lobectomy, and pulmonary artery angioplasty (6,7). Jutley et al. reported that single-port VATS could decrease postoperative pain after pneumothorax surgery (8). Gonzalez-Rivas further reported that single-port VATS does not increase the rate of postoperative complications and death when compared with multiple-port VATS (9). Liu reported that, in the lobectomy, single-port VATS has other advantages, including shorter surgery time, less blood loss and more resected lymph nodes (10). VATS-assisted lobectomy is widely used to treat NSCLC. Gonzalez-Rivas studied this treatment for advanced cases of NSCLC using uni-port VATS, and the results showed that this was feasible (11).
There are still some undetermined issues regarding the single-port VATS such as the position of the incision, the incision size and the instrument (5,7). To our knowledge, there is no report concerning the comparison between the single-port VATS and two-port VATS in treating NSCLC. Therefore, we conducted this study to compare the efficacy between these two methods in treating NSCLC. To avoid selection bias and the influence of intraoperative variations, a propensity-matched analysis was performed. We retrospectively analyzed and compared the perioperative outcomes and the short-term follow-up results between the two treatment methods.
Methods
Patients
This study was approved by the ethic committee of Daping Hospital & Institute of Surgery Research, the Third Military Medical University (ID of the approval: 2015-No.54), and the signed informed consent was obtained from each patient. We performed a retrospective evaluation on the clinical data collected from patients undergoing either single-port VATS or two-port VATS for NSCLC from January 2013 to June 2015. The inclusion criteria were NSCLC, peripheral lung cancer, clinical staging of T1–T3 and N0–N2, and no distant metastases. The exclusion criteria were benign lung lesions; cT3 tumors invading to the chest wall, diaphragm or pericardium; sub-pulmonary lobectomy and preoperative neoadjuvant therapy.
Patient data, including demographics, pulmonary function [e.g., forced vital capacity; forced expiratory volume in 1 second (FEV1)], operative results, pathologic reports, and in-hospital morbidity and mortality were collected. The patients’ comorbidities were assessed using the Charlson comorbidity index (CCI) (12). The postoperative complications were evaluated by the Common Terminology Criteria for Adverse Events (13). The death percentage was defined as “patients who died” during the hospital stay or within 30 postoperative days. The histological type was evaluated according to the World Health Organization classification of lung cancer (14). The tumor stage was determined according to the American Joint Committee on Cancer staging system, 7th edition (15).
Treatment method
All patients underwent pulmonary function testing, routine chest enhanced-computed tomography (enhanced-CT) (GE Company, Connecticut, USA) and integrated positron emission tomography (PET)/CT (Siemens, Munich, Germany) to determine the tumor staging. Fiber bronchoscopy, routine blood examination and blood biochemical examination were also performed.
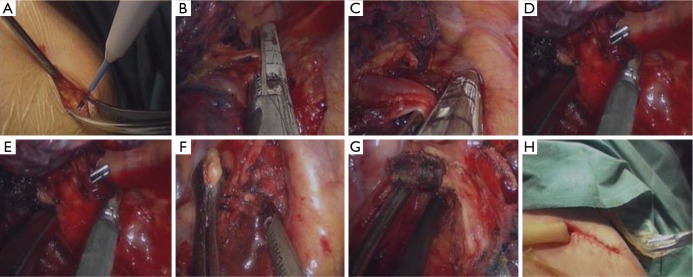
Patients were divided into two groups: one group that underwent single-port VATS and another group that underwent two-port VATS. The surgeries were completed by the same doctor. All of the patients underwent general anesthesia in the lateral decubitus position with single lung ventilation using double lumen endotracheal intubation. For the two-port VATS, a 4–5 cm anterior axillary incision was made in the fourth intercostal space and used as the utility port. The serratus anterior was split along the muscle fibers without rib spreading. A lap-protector was placed to avoid operative damage to the intercostal muscles and intercostal nerve. An axillary midline incision (1.0 cm) was made in the seventh intercostal space and used as the thoracoscopy port. A thoracoscope (10 mm, 30°; Stryker Corporation, Michigan, USA) was inserted. For the single-port VATS, an axillary midline incision (3–5 cm) (Figure 1A) was made in the fifth intercostal space along the front latissimus dorsi. The first assistant stood at the back of the patient and attempted to keep the thoracoscope at the posterior end of the incision. The surgeon was positioned in front of the patient with enough space for the surgeon to operate. The lobectomy was performed similarly in the two groups. The pulmonary vein, pulmonary artery, and bronchus were ligated and divided with an endoscopic stapler (Medtronic Co., Minnesota, USA) (Figure 1B,C,D,E). The resected lung tissue was placed into a small bag and removed from the operation hole. A conventional systematic mediastinal lymph node resection was performed. The resected lymph nodes were of stations 2, 3, 4, 7, 8 and 9 in the right lung cancers and of stations 4L, 5, 6, 7, 8 and 9 in the left lung cancers (Figure 1F,G). In the two-port VATS, a chest drainage tube (MD-45110, Akita Sumitomo Bakelite Co. Ltd., Akita, Japan) was placed through the observational hole, reached the apex of the thorax and did not contact the subclavian vessels. In the single-port VATS, a chest drainage tube was placed through the posterior border of the incision (Figure 1H). The muscular layer and subcutaneous tissue around the drainage tube were carefully stitched in case of postoperative fluid leakage. The tube was removed depending on the drainage. The postoperative analgesia was performed by intravenously administering 150 mL normal saline (0.9%), 50 μg sufentanil, 150 mg dezocine and 8 mg ondansetron hydrochloride for 48 hours.
Figure 1.
Single-port VATS in a patient with non-small cell lung cancer. (A) An axillary midline incision (3–5 cm) was made in the fifth intercostal space; (B-E) the right upper pulmonary vein (B), right upper pulmonary artery (C,E) and right upper bronchus (D) were ligated and divided using an endoscopic stapler; (F,G) the resected lymph nodes were of station 2 and 4 (F) and 7 (G); (H) a chest drainage tube was placed through the posterior border of the incision. VATS, video-assisted thoracic surgery.
Follow-up
The postoperative pain was recorded 1 and 3 days postoperatively using the visual analogue scale (VAS) [0–10]. The score of the patient satisfaction [0–100], which was measured by using the VAS (satisfaction scale), was recorded on the discharge day. The patients were followed up every month within the postoperative 6 months.
Statistical analysis
Qualitative variables are expressed as a number (percentage) and were analyzed by either chi-square test or Fisher’s exact test. The quantitative variables are expressed as the mean ± standard deviation and analyzed by the two-tailed t-test. P<0.05 was considered to be statistically significant. SPSS 17.0 was used.
Propensity score (PS) analysis was conducted using logistic regression to create a PS for individual patients using demographic and clinical variables. The variables used to estimate the PS were age, gender, BMI, FEV1 and the percentage of its predicted value, the CCI and the size of the tumor. The PS was calculated using a logistic model. Each patient who underwent two-port VATS was matched with a patient who underwent single-port VATS and had the closest estimated PS.
Results
Patient characteristics
Of the 143 patients who met the inclusion criteria, 66 (66/143, 46.2%) underwent two-port VATS and 77 (77/143, 53.8%) underwent single-port VATS. The patients’ baseline characteristics are presented in Table 1.
Table 1. Patients’ baseline and clinical characteristics.
| Characteristics | Unmatched patients | Matched patients | |||||
|---|---|---|---|---|---|---|---|
| Two ports (n=66) | Single port (n=77) | P value | Two ports (n=63) | Single port (n=63) | P value | ||
| Age (years) | 56.50±12.58 | 58.27±9.21 | 0.334 | 57.11±12.22 | 58.68±9.24 | 0.417 | |
| Female (%) | 18 (27.27) | 32 (41.56) | 0.081 | 17 (26.98) | 23 (36.51) | 0.339 | |
| BMI (kg/m2) | 22.44±2.89 | 21.86±3.13 | 0.254 | 22.04±3.01 | 21.99±3.06 | 0.919 | |
| FEV1 (L) | 1.98±0.38 | 2.05±0.45 | 0.320 | 1.98±0.38 | 1.99±0.40 | 0.883 | |
| Predicted FEV1 (%) | 84.38±15.53 | 86.06±14.91 | 0.511 | 84.28±15.83 | 85.05±14.85 | 0.779 | |
| CCI | 1.68±1.30 | 1.74±1.14 | 0.775 | 1.67±1.28 | 1.73±1.09 | 0.766 | |
| Tumor size (cm) | 4.28±1.67 | 3.83±1.61 | 0.103 | 4.21±1.66 | 3.92±1.57 | 0.314 | |
| Cell type (%) | 0.661 | 0.633 | |||||
| Adenocarcinoma | 53 (80.30) | 65 (84.42) | 51 (80.95) | 54 (85.71) | |||
| Squamous cell | 12 (18.18) | 12 (15.58) | 12 (19.05) | 9 (14.29) | |||
| Carcinoid tumor | 1 (1.52) | 0 | 0 | 0 | |||
| Pathological stage (%) | 0.222 | 0.750 | |||||
| Ia | 7 (10.61) | 16 (20.78) | 7 (11.11) | 10 (15.87) | |||
| Ib | 25 (37.88) | 23 (29.87) | 23 (36.51) | 20 (31.75) | |||
| IIa | 12 (18.18) | 11 (14.29) | 12 (19.05) | 11 (17.46) | |||
| IIb | 2 (3.03) | 8 (10.39) | 2 (3.17) | 5 (7.94) | |||
| IIIa | 19 (28.79) | 17 (22.08) | 18 (28.57) | 15 (23.81) | |||
| IIIa | 1 (1.51) | 2 (2.59) | 1 (1.59) | 2 (3.17) | |||
FEV1, forced expiratory volume in 1 second; CCI, Charlson comorbidity index.
PS estimation
After 1-to-1 PS matching, 63 pairs were selected. Table 1 summarizes the baseline patient characteristics of the two study groups. Both groups were well balanced for age, gender, body mass index, pulmonary function, preoperative comorbidity, tumor size and tumor type. The majority of the patients were male. The cell types of the tumors were similar between the two groups, with most patients diagnosed with adenocarcinoma.
Treatment results
Patients who underwent single-port VATS had less blood loss (P=0.006), less postoperative pain (P=0.001), and a higher satisfaction score (P=0.049) than those who underwent two-port VATS (Table 2). Postoperative complications occurred in 2/63 (3.2%) of patients in the single-port VATS group and 6/63 (9.5%) of patients in the two-port VATS group without any significant differences. There was one case of diarrhea and one case of pleural fluid leakage in the single-port VATS group, and there were three cases of pleural fluid leakage, one case of incision infection, one case of pneumothorax and one case of atrial fibrillation in the two-port VATS group. The mean follow-up was 6 months for the two groups. No deaths occurred during the follow-up period.
Table 2. The comparison of the treatment results between the two groups.
| Results | Unmatched patients | Matched patients | |||||
|---|---|---|---|---|---|---|---|
| Two ports (n=66) | Single port (n=77) | P | Two ports (n=63) | Single port (n=63) | P | ||
| Surgery time (min) | 176.70±57.70 | 180.50±38.10 | 0.635 | 177.70±58.20 | 184.90±39.50 | 0.418 | |
| Blood loss (mL) | 252.70±189.80 | 175.80±107.10 | 0.012* | 260.80±190.00 | 178.90±114.60 | 0.006* | |
| Resection of lymph nodes | 17.90±6.70 | 17.00±6.10 | 0.501 | 17.10±6.60 | 16.30±5.90 | 0.431 | |
| Drainage (days) | 4.36±3.48 | 4.36±2.67 | 0.228 | 4.44±3.54 | 4.54±2.87 | 0.195 | |
| VAS score POD 1 | 5.92±1.35 | 5.19±1.41 | 0.020* | 5.90±1.33 | 5.14±1.24 | 0.001* | |
| VAS score POD 3 | 3.00±0.84 | 2.16±0.88 | 0.002* | 3.03±0.82 | 2.13±0.85 | 0.001* | |
| Satisfaction score | 92.06±7.26 | 94.77±5.48 | 0.012* | 92.00±7.39 | 94.59±5.69 | 0.029* | |
| Hospital stay | 8.18±4.07 | 8.12±3.43 | 0.742 | 8.30±4.11 | 8.35±3.66 | 0.703 | |
| Complication rate (%) | 6 (9.10) | 4 (5.19) | 0.513 | 6 (9.52) | 2 (3.17) | 0.273 | |
*, indicates a statistical significance. VAS, visual analogue scale.
The single-port VATS group was further analyzed (Table 3). Compared with the middle/lower lobectomy, the upper lobectomy had a longer drainage time (5.00±3.43 vs. 3.68±1.16; P=0.029). Patients with larger tumors required a longer surgery time (P<0.05) and had more blood loss, especially when the tumor size >5 cm (P<0.05).
Table 3. The clinical results of the single-port video-assisted thoracic surgery group.
| Characteristics | Number/percent (%) | Surgery time (min) | Blood loss (mL) | Resection of lymph nodes | Drainage time (days) | Hospital stay (days) |
|---|---|---|---|---|---|---|
| Tumor site | ||||||
| Upper lobe | 40 (51.9) | 181.9±38.5 | 167.0±110.8 | 17.6±6.5 | 5.0±3.4 | 8.2±3.8 |
| Middle/lower lobe | 37 (48.1) | 179.1±38.1 | 185.4±103.5 | 15.9±5.7 | 3.7±1.2 | 8.1±3.1 |
| P value | – | 0.748 | 0.455 | 0.310 | 0.029* | 0.930 |
| Tumor size (cm) | ||||||
| ≤3 | 27 (35.1) | 168.2±39.4 | 167.8±100.7 | 16.8±5.3 | 3.9±2.7 | 7.9±3.1 |
| >3 | 50 (64.9) | 187.2±36.1 | 180.2±111.1 | 16.8±6.6 | 4.6±2.8 | 8.3±3.6 |
| P value | – | 0.035* | 0.630 | 0.762 | 0.254 | 0.621 |
| Tumor size (cm) | ||||||
| ≤5 | 57 (74.0) | 173.9±32.8 | 157.0±86.4 | 17.2±6.2 | 4.3±2.7 | 7.8±3.1 |
| >5 | 20 (26.0) | 199.5±46.1 | 229.5±140.7 | 15.1±5.7 | 4.6±2.8 | 9.1±4.1 |
| P value | – | 0.009* | 0.008* | 0.174 | 0.719 | 0.158 |
*, indicates a statistical significance.
Discussion
The present study showed that single-port VATS had better results compared with the two-port VATS, including less blood loss, less postoperative pain and higher patient satisfaction scores.
Liu et al. reported that a single-port VATS lobectomy has many advantages, including shorter surgery time and more resected lymph nodes compared with multiple-port VATS (10). Kuritzky et al. reported that VATS could resect a similar number of lymph nodes compared with open thoracic surgery. Our study showed that there was no significant difference in the surgery time between the two groups. We thought that it was probably due to the small incision in the single-port VATS; the operating room for the surgeon was actually very small as the thoracoscope occupied approximately 10 mm. This inevitably resulted in limited operational space, although we used certain special equipment to reduce the mutual influence between them. Moreover, Gonzalez-Rivas reported that single-port VATS required the experience of a double-port lobectomy and a simple single-port chest surgery. He also emphasized the importance of sufficient exposure, the proper placement angle of the linear cutter, and minimal interference of both the thoracoscope and the instrument on the interior and exterior of the thoracic cavity (16). As for the resection of the mediastinal lymph node, there was no significant difference between the two groups. The present study adopted a systemic lymph node cleaning method considering that this method may affect the postoperative pathological staging and long-term prognosis. The blood loss was lower in the single-port VATS group, which was probably due to the experience and the good cooperation of the fixed surgical team.
The VAS score was lower in the single-port VATS group, which may be attributed to reduced intercostal nerve injury and only one incision in the single-port VATS procedure. This result is in accordance with the previous study. For example, Mier et al. (17) compared the results of a single-incision thoracoscope and three-incision thoracoscope and demonstrated that the single-hole group had a lower pain score. In the single-port VATS group, the satisfaction score was significantly higher than that of the two-port VATS group. This is probably because the single-port VATS is cosmetically appealing with less postoperative pain. There was no significant difference in chest drainage duration, the incidence of complications and the length of hospital stay between the two groups.
As for the incision in the single-port VATS group, we made a 4.0 cm incision in the fifth intercostal space between the midaxillary line and anterior axillary line ahead of the latissimus dorsi and along the serratus anterior. It was performed without rib spreading. A lap-protector was used to protect the chest wall from the instrument. The incision position in our study is different from other reports (7). The incision in the present study is advantageous for conversion to open thoracotomy, which occurs by extending the existing incision and continuing the surgery and is relatively rapid and convenient (18).
Compared with the middle/lower lobectomy, the upper lobectomy required a longer drainage time. The lung would fall postoperatively because of gravity, which also hindered pulmonary re-expansion. It takes time for a complete pulmonary re-expansion. The re-expansion of the residual cavity depends on the expansion of the pulmonary lobe and the uplifting of the diaphragm after lung lobectomy. After lower lobe resection, the expansion of the upper lung lobe remained in its original position while the position of the bronchus did not change much. On the contrary, after upper lobe resection, the lower lobe shifted upward and expanded; this process had to overcome gravity and required a positional change of the lower lobe bronchus as reported by Seok et al. (19). However, Seok et al. did not note that there was significant difference in the drainage time between upper lobe resection and lower lobe resection in their study. This is highly controversial regarding the drainage time. Kouritas et al. reported that lower lobectomy required a longer drainage time compared with upper lobectomy (20). Currently we are studying this phenomenon to find the reason and reduce the drainage time. According to our clinical experience, we propose changing the patient’s posture and deep breathing training in our on-going study. We hope to provide more details in our continuing research.
There are some limitations in the present stud, such as the retrospective nature and short-term follow-up period. In conclusion, single-port VATS lobectomy is suggested to be safe and feasible for treating NSCLC. Compared with two-port VATS, single-port VATS has many advantages, including less blood loss, less postoperative pain and higher satisfaction score.
Acknowledgements
None.
Ethical Statement: This study was approved by the ethic committee of Daping Hospital & Institute of Surgery Research, the Third Military Medical University (ID of the approval: 2015-No.54), and the signed informed consent was obtained from each patient.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011;378:1727-40. 10.1016/S0140-6736(10)62101-0 [DOI] [PubMed] [Google Scholar]
- 2.Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. 10.1093/icvts/ivs472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. 10.1016/j.jtcvs.2009.11.059 [DOI] [PubMed] [Google Scholar]
- 4.Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. 10.1016/j.athoracsur.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Rivas D. Single-port video-assisted thoracoscopic lobectomy. In: Inderbitzi RG, Schmid RA, Melfi FM, et al. editors. Minimally Invasive Thoracic and Cardiac Surgery. Modena: Springer, 2012:105-11. [Google Scholar]
- 6.Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. 10.1097/SLA.0000000000000712 [DOI] [PubMed] [Google Scholar]
- 8.Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. 10.1016/j.ejcts.2005.02.039 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. 10.1016/j.athoracsur.2012.10.070 [DOI] [PubMed] [Google Scholar]
- 10.Liu CC, Shih CS, Pennarun N, et al. Transition from a multiport technique to a single-port technique for lung cancer surgery: is lymph node dissection inferior using the single-port technique?†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i64-72. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 13.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. 10.1016/S1053-4296(03)00031-6 [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 15.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Rivas D. Evolving thoracic surgery: from open surgery to single port thoracoscopic surgery and future robotic. Chin J Cancer Res 2013;25:4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mier JM, Chavarin A, Izquierdo-Vidal C, et al. A prospective study comparing three-port video-assisted thoracoscopy with the single-incision laparoscopic surgery (SILS) port and instruments for the video thoracoscopic approach: a pilot study. Surg Endosc 2013;27:2557-60. 10.1007/s00464-012-2782-6 [DOI] [PubMed] [Google Scholar]
- 18.Anile M, Diso D, Mantovani S, et al. Uniportal video assisted thoracoscopic lobectomy: going directly from open surgery to a single port approach. J Thorac Dis 2014;6:S641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seok Y, Yi E, Cho S, et al. Perioperative outcomes of upper lobectomy according to preservation or division of the inferior pulmonary ligament. J Thorac Dis 2015;7:2033-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouritas VK, Zissis C, Bellenis I. Variation of the postoperative fluid drainage according to the type of lobectomy. Interact Cardiovasc Thorac Surg 2013;16:437-40. 10.1093/icvts/ivs529 [DOI] [PMC free article] [PubMed] [Google Scholar]