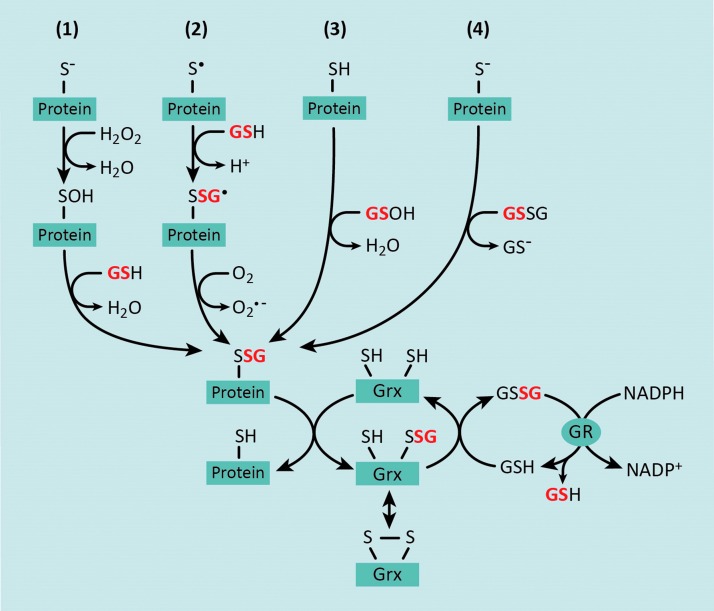
FIG. 1.
Biochemical mechanisms for reversible S-glutathionylation of protein thiols. There are four major mechanisms by which protein thiols can become S-glutathionylated: (1) in the presence of hydrogen peroxide (H2O2), protein thiols are oxidized to highly reactive protein sulfenic acids that can react with the glutathione (GSH); (2) several lines of evidence support a thiyl radical-mediated mechanism for protein-S-glutathionylation, which involves a protein thiyl radical (protein-S·) reacting with GSH and being stabilized by radical transfer to molecular oxygen to form superoxide (O2−•); (3) cellular GSH can react with endogenous reactive oxygen species (ROS) to form a GSH sulfenic acid, thereby facilitating a reaction with a reduced protein thiol; and (4) GSH exchange occurs between glutathione disulfide (GSSG) and a protein thiolate, generating an S-glutathionylated protein and GSH. Protein-S-glutathionylation is reversed by the activity of glutaredoxin (Grx) enzymes. Deglutathionylation and reduction of a target protein involve the transfer of the glutathionyl group onto Grx, which can subsequently be reduced by GSH, resulting in the formation of GSSG. In turn, GSSG is reduced back to two molecules of GSH by glutathione reductase (GR), which uses electrons from NADPH. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars