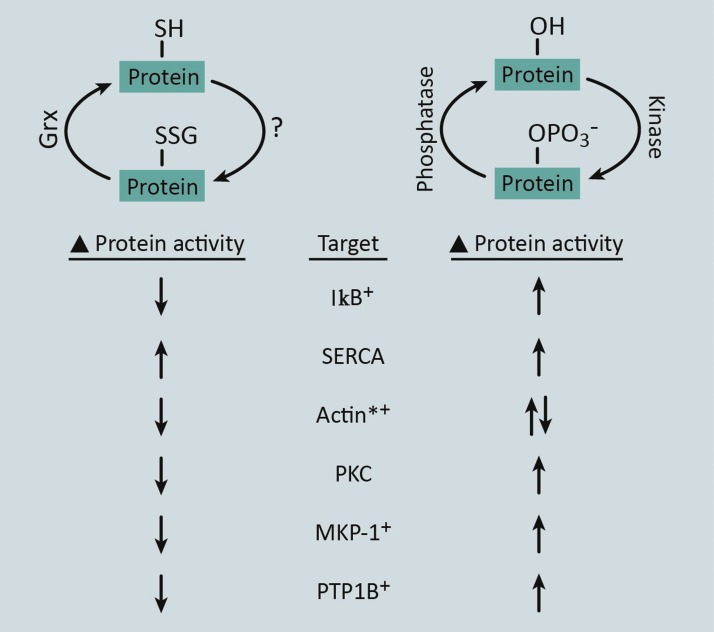
FIG. 2.
Proteins regulated by both protein-S-glutathionylation and protein phosphorylation. The reversible nature of protein-S-glutathionylation resembles the process of protein phosphorylation. The forward mechanism of protein phosphorylation is driven by kinases, whereas the exact mechanism of protein-S-glutathionylation in intact cells and tissues is not yet fully understood (?). Depending on the system, protein-S-glutathionylation appears to be driven by either nonenzymatic or enzymatic mechanisms. The reversal of phosphorylation is mediated by phosphatase enzymes, whereas S-glutathionylation is reversed by Grx enzymes. In addition, both of these modifications have been reported to alter the activity of the proteins (examples are listed: Target), although via modification of discrete amino acids. *Phosphorylation and glutathionylation of actin result in changes in polymerization state, as opposed to changes in enzymatic activity for the other protein targets. +Proteins for which S-glutathionylation has been reported in monocytes and/or macrophages. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars