Abstract
We report a very rare case of Sevoflurane Induced Diffuse Alveolar Hemorrhage in a previously healthy young adult in the post-operative period following general anesthesia. Diffuse alveolar hemorrhage (DAH) associated with inhalation injury from halogenated gases is a unique entity in the literature that practicing clinicians should be cognizant of and considered in post-operative cases of acute respiratory distress whereby other etiologies have been excluded.
Keywords: Inhaled anesthetics, Pulmonary hemorrhage, Acute respiratory distress
1. Introduction
Diffuse alveolar hemorrhage (DAH) results from widespread damage to the pulmonary small vessels, leading to blood collecting within the alveoli. In this report, we describe a novel case of an inhaled anesthetic, Sevoflurane, inducing pulmonary hemorrhage in an otherwise healthy individual during a routine urological procedure.
2. Clinical case
A 20-year-old man was admitted for a urethral stricture dilation via cystoscopy and retrograde urethrogram. He had a history of exercise-induced asthma.
His preoperative anesthesia evaluation was unremarkable. Induction medications included intravenous propofol, fentanyl and versed. The surgery was performed under general anesthesia using inhaled sevoflurane.
The patient was successfully extubated following the surgery. However, approximately one hour after the completion of the procedure, he developed acute hypoxemic respiratory distress in the post-anesthesia care unit requiring reintubation.
Postoperatively, the patient was afebrile, with heart rate 115 beats/min, BP of 95/50 mm Hg, respiratory rate of 22 breaths/min, and an oxygen saturation of 91% on 35% 40 L/min via high flow nasal cannula.
Chest examination was remarkable for coarse crackles that were most prominent at the bases of the chest bilaterally. The remainder of the examination was normal.
A hemogram demonstrated a leukocytosis (13.6 × 109/L), a normal hemoglobin level (14.0 g/dL) and platelet count (157 × 109/L). He had a normal serum creatinine (1.1 mg/dL). An echocardiogram demonstrated normal left ventricular function, and no structural or valvular abnormalities.
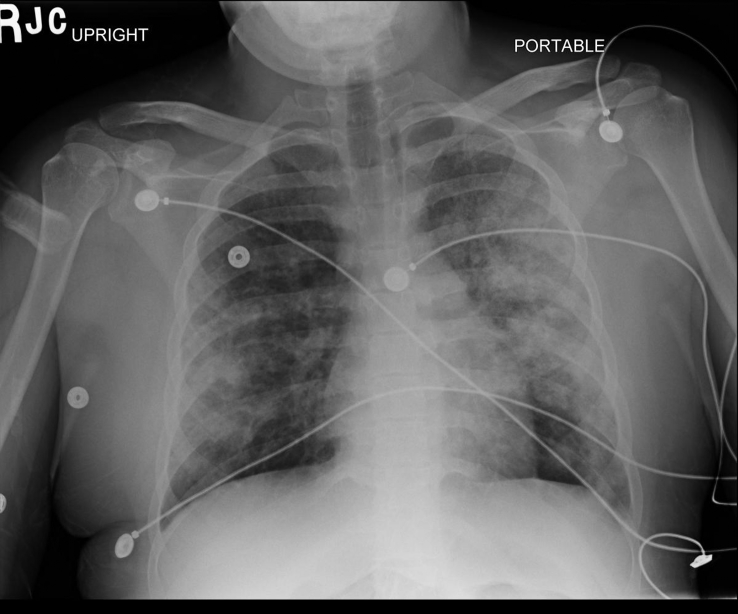
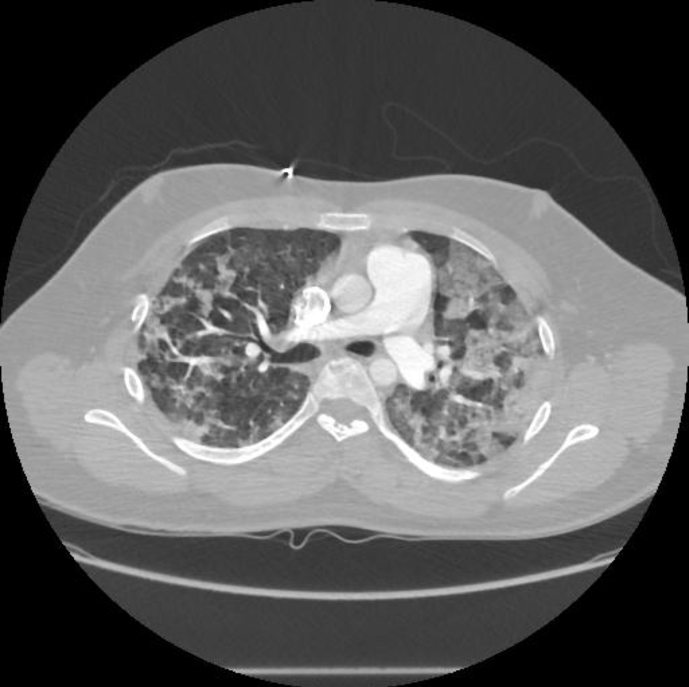
Pre-procedure, chest radiography (CXR) was unremarkable; however postoperatively, the CXR demonstrated patchy, peripheral consolidations bilaterally, sparing the apices and bases (Fig. 1). Computer tomography of the thorax revealed patchy bilateral lung consolidation and ground glass opacities in an upper lobe predominant distribution (Fig. 2). Given high index of suspicion for pulmonary hemorrhage, the patient underwent a diagnostic bedside bronchoscopy, which demonstrated diffuse erythema throughout the tracheobronchial tree. There was no evidence of thermal airway injury. Sequential bronchoalveolar lavage of multiple lobes (anterior segment of the right and left upper lung lobes) showed progressively bloody return consistent with diffuse alveolar hemorrhage.
Fig. 1.
CXR PA View- Demonstrating Bilateral Alveolar Infiltrates without effusion.
Fig. 2.
CT chest sagittal section- demonstrating bilateral, peripheral alveolar opacities.
Microbiological workup for bacterial, fungal and viral infections were non-revealing. Extensive serological workup for vasculitides was also negative.
The patient was managed conservatively with supportive care and improved clinically with successful extubation in three days. Follow up CXR at one month showed complete resolution of pulmonary infiltrates.
3. Discussion
Sevoflurane is a commonly used volatile inhaled halogenated gas for rapid induction during general anesthesia. The medication has a rapid washout time in comparison to other agents in its class due to its lower blood solubility and blood–gas partition coefficient [1].
Sevoflurane is metabolized directly by the two forms of alkaline carbon dioxide absorbents in the circuits of anesthesia machines producing toxic metabolites. The main metabolite is fluoromethyl-2,2-difluoro-1-(trifluoromethyl) vinyl ether, referred to as Compound A [2]. Compound A has been hypothesized to cause direct renal toxicity in murine and human studies [3], [4]. It is unclear if Compound A causes pulmonary toxicity. However, pulmonary endothelial damage associated with other halogenated gases has been postulated in the literature. Volatile gases are lipid soluble and potentiate the arachidonic cascade in the cell membrane [4]. As a consequence, exposure can cause alveolar permeability, increased oxidative stress and heightened inflammatory response [5]. Sevoflurane may also activate a similar pathway in the appropriate setting.
Several case studies reported instances of exothermic reactions with sevoflurane and the carbon dioxide absorbents in the anesthesia circuits producing fire resulting in thermal airway injuries and acute respiratory distress syndrome from spontaneous combustion [6], [7]. Possibly, occult less severe thermal injuries may be responsible for previous reports of pulmonary toxicity from Compound A.
To date, only two cases have been reported describing the development of pulmonary hemorrhage following sevoflurane administration during general anesthesia [8], [9]. Airway inspection in the both reports did not demonstrate signs of thermal injury. There was no evidence of an alternative cause of lung injury including infectious, inflammatory or malignant etiologies. Treatment in both cases was supportive and there was no recurrence of pulmonary injury.
In conclusion, DAH from inhaled sevoflurane should be considered in post-operative cases of acute respiratory distress and pulmonary hemorrhage in which other etiologies have been excluded.
Conflicts of interest
All Authors have no disclosures or conflicts of interest, and there were no sources of funding for this manuscript.
Acknowledgements
Adam Austin is the leading author of the manuscript. He was responsible in part for the concept, writing, content review, and editing for this manuscript.
Aakash Modi, Marc A. Judson and Amit Chopra were contributing authors of the manuscript. They are authors of the manuscript and have had content review and editing input for the manuscript.
References
- 1.Oladay D.A., Smith F.R. Clinical characteristics and biotransformation of sevoflurane in healthy human volunteers. Anesthesiology. 1981;54:100–160. doi: 10.1097/00000542-198102000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Behne M., Wilke H.J., Harder S. Clinical pharmacokinetics of sevoflurane. Clin. Pharmoacokinet. 1999;36(1):13–26. doi: 10.2165/00003088-199936010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Gonsowski C.T., Laster M.J., Eger E.I., II Toxicity of compound A in rats: effect of a 3-hour administration. Anesthesiology. 1994;80:556–565. doi: 10.1097/00000542-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Gentz B.A., Malan T.P. Renal toxicity with sevoflurane: a storm in a teacup? Drugs. 2001;61(15):2155–2161. doi: 10.2165/00003495-200161150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Shayevitz J.R., Traystman R.J., Adkinson N.F., Sciuto A.M., Gurtner G.H. Inhalation anesthetics augment oxidant-induced pulmonary vasoconstriction: evidence for a membrane effect. Anesthesiology. 1985;63:624–632. doi: 10.1097/00000542-198512000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Fatheree R.S., Leighton B.L. Acute respiratory distress syndrome after an exothermic baralyme-sevoflurane reaction. Anesthesiology. 2004;101:531–533. doi: 10.1097/00000542-200408000-00034. [DOI] [PubMed] [Google Scholar]
- 7.Wu J., Previte J.P., Adler E., Myers R., Ball J., Gunter J.B. Spontaneous ignition, explosion, and fire with sevoflurane and barium hydroxide lime. Anesthesiology. 2004;101:534–537. doi: 10.1097/00000542-200408000-00035. [DOI] [PubMed] [Google Scholar]
- 8.Khanna A.K., Cummings K.C., III Pulmonary hemorrhage in an outpatient ophthalmic anesthesia setting- it's never “just a cataract.”. J. Anaesthesiol. Clin. Pharmacol. 2012;28(4):520–523. doi: 10.4103/0970-9185.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim C.A., Liu R., Hsia D.W. Diffuse alveolar hemorrhage induced by sevoflurane. Am. Thorac. Soc. 2014;11(5):853–855. doi: 10.1513/AnnalsATS.201402-067LE. [DOI] [PubMed] [Google Scholar]