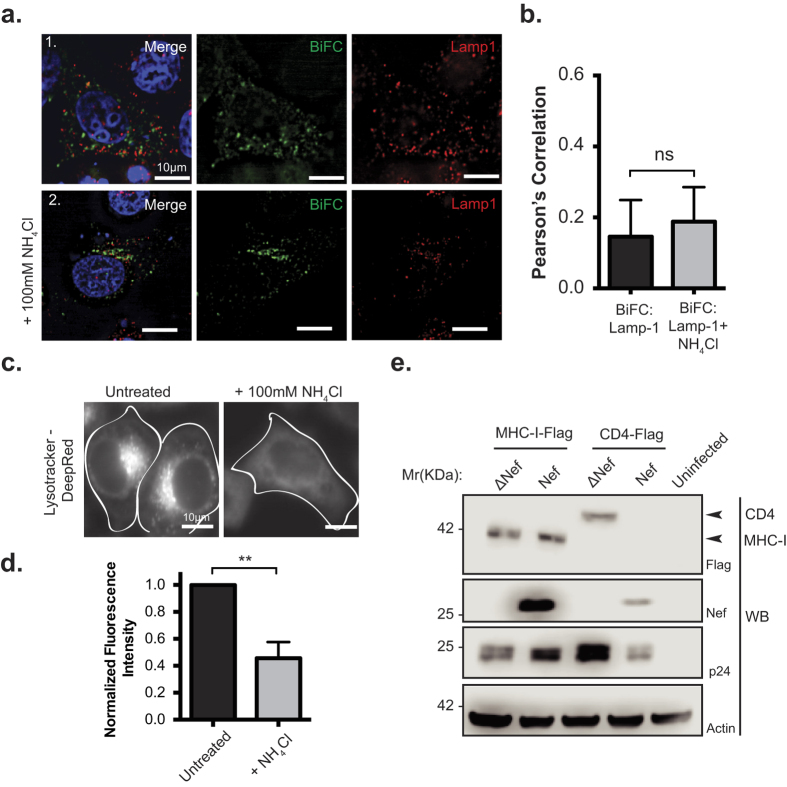
Figure 5. Nef:MHC-I interaction does not occur in lysosomes.
(a) HeLa cells were co-transfected with Nef-VC and MHC-I-VN-Flag constructs and immunostained for LAMP-1 (Panel 1), or, treated with 100 mM ammonium chloride (NH4Cl) for 3 hours prior to fixation and staining (Panel 2). BiFC fluorescence (green) was visualized under the FITC channel, whereas LAMP-1 stain was visualized under the Cy5 filter settings, and pseudo-colored red. Nuclei were stained with DAPI, and scale bars represent 10 μm. (b) Co-localization was quantified by the Pearson’s Correlation through the JaCoP Plug-in on ImageJ. Error bars were calculated by quantification of at least 25 cells between 3 independent experiments. (c,d) HeLa cells were treated with PBS, or 100 mM of ammonium chloride for 3 hours, and then treated with 10 μM Lysotracker Deep Red for 5 minutes. Live cells were then imaged at 37 °C in 5% CO2 and quantified for Lystotracker fluorescence. Error bars were calculated by quantification of at least 25 cells between 3 independent experiments. (**Indicates p-value < 0.01). (e) Sup-T1 cells were infected with F2A MHC-I-Flag-Nef/ΔNef or F2A-CD4-Flag-ΔVpu-Nef/ΔNef viruses. 48 hours post infection, cells were lysed and analyzed by Western blot. Anti-Flag detected total MHC-I-Flag and CD4-Flag, whereas anti-Nef antibody marked the presence or absence of Nef. Anti-p24 and anti-actin antibodies were used as infection and loading controls, respectively. A representative Western blot from 3 independent experiments is shown.