Abstract
Context:
Spinal dysraphisms are congenital abnormalities of the spine due to imperfect fusion of midline mesenchymal, bony and neural structures. Imaging plays a vital role in their evaluation as significant portion of patients may present with concurrent anomalies that need to be corrected simultaneously to avoid repeat surgeries.
Aims:
The aims of the study were to evaluate Spinal dysraphisms using USG and MRI and to correlate imaging findings with operative findings in patients undergoing surgery.
Settings and Design:
Hospital based observational study conducted over a period of year.
Materials and Methods:
38 cases of both sexes and below 12 years of age with spinal dysraphism were studied. USG was performed in 29 cases where acoustic window was available for proper evaluation. MRI was performed in all cases. USG findings were compared with MRI findings and operative follow up was taken in 23 cases who underwent operative management.
Statistical Analysis Used:
Results were analysed using percentage and arithmetic mean.
Results:
39.47 % cases were male and 60.53 % cases were female. Neonatal period was the most common presenting age group. Closed spinal dysraphism (63.16%) was more common than open (36.84%). 79.31% cases showed full agreement between spinal USG and MRI examinations and 6 out of 20.69% showed partial agreement. On operative correlation, USG findings were confirmatory in 91.30% cases and MRI findings were confirmatory in 100% cases.
Conclusions:
USG can be used as the initial modality for evaluation of spinal dysraphism as well as for screening of suspected cases. MRI is indicated to confirm abnormal USG findings, which shows all concurrent abnormalities and also provides additional anatomical details relevant to surgical planning.
Key words: Congenital spinal anomalies, magnetic resonance imaging, spinal dysraphisms, spinal ultrasonography, tethered cord
Introduction
Congenital malformations of the spinal cord are collectively termed spinal dysraphisms (SDs).[1] These conditions are usually diagnosed at birth or in early infancy, but some may be discovered in older children or adults. The estimated incidence of SD is about 1–3/1000 live births.[2]
SDs are categorized into open SDs (OSDs), in which there is an exposure of abnormal nervous tissues through a skin defect, and closed SDs (CSDs), in which there is continuous skin coverage to the underlying malformation.[3]
OSD includes meningomyelocele (MMC) and other rare abnormalities such as myelocele, hemimyelomeningocele, and hemimyelocele are always associated with a Chiari II malformation.
CSDs are further divided into two subsets based on whether a subcutaneous mass is present in the low back. CSD with mass comprises lipomyelocele, LipoMMC, meningocele, and myelocystocele. CSD without mass comprises simple dysraphic states (tight filum terminale, filar and intradural lipomas, persistent terminal ventricle, and dermal sinuses) and complex dysraphic states. The latter category involves abnormal notochordal development, either in the form of failed midline integration (ranging from complete dorsal enteric fistula to neurenteric cysts and diastematomyelia) or in the form of segmental agenesis (caudal agenesis and spinal segmental dysgenesis).
It is possible to perform spinal ultrasonography (USG) in the newborn and early infants, owing to the lack of ossification of the predominantly cartilaginous posterior elements of the spine. Spinal USG is usually not possible after the age of 6 months except in cases of a persistent posterior spinal defect.
Magnetic resonance imaging (MRI) is extremely useful for the evaluation of spinal anomalies. Due to its multiplanar capabilities, the lack of ionizing radiation, and its superior soft-tissue contrast, MRI allows for the delineation of the spinal cord, the subarachnoid space, the vertebral bodies, and the intervertebral discs and can be employed in infants and children without harmful effects of radiation. It has greatly improved the diagnosis of these disorders and has enhanced the possibility of earlier and case-tailored treatment. Thus, MRI is superior to other modalities in exactly defining spinal dysraphic lesions and is the imaging modality of choice for evaluation of this complex group of disorders.
We conducted the study to determine the role of imaging in the evaluation of SDs, which would help in better management and prognostication of these patients.
The aims of the study were as follows:
To evaluate SDs by USG and MRI
To compare the findings of both the modalities in patients undergoing both spinal USG and MRI
To correlate imaging findings with operative findings in patients undergoing surgery.
Subjects and Methods
After ethical approval from the Institutional Review Board, we conducted a hospital-based observational study on 38 patients over the period from July 2014 to June 2015. The study comprised patients of both sexes and below 16 years of age having clinical features related to SD.
Inclusion criteria
All cases with clinical diagnosis of SD
All incidentally detected cases of SD
Children with anorectal malformation.
Exclusion criteria
Postoperative cases of SD.
Spinal ultrasonography
Spinal USG was performed in all infants and in children where adequate USG evaluation of back swelling and/or spinal cord was possible without posterior acoustic shadowing due to a persistent defect in the posterior elements (spina bifida). In older children, USG evaluation was not possible due to posterior acoustic shadowing from ossified posterior elements. Hence, USG examination of the spine was not performed in these older patients. The patients were examined using Siemens Acuson Antares 5 Ultrasound System (Siemens Healthcare, Erlangen, Germany) with the use of 7.0–12.0 MHz real-time high-frequency linear array transducer. The examination was done in both sagittal and axial planes along the entire spine with patients in prone or lateral decubitus position. Gray scale examination was done in all cases with the addition of color Doppler interrogation as and when required.
Magnetic resonance imaging
MRI examination of the spine was performed in all patients and screening of brain was done with limited sequences, in cases where associated brain anomaly was suspected (e.g., Arnold–Chiari malformation II [ACM II]). Infants and young children were sedated to keep them quiet during scanning by giving oral chloral hydrate syrup or intravenous lorazepam.
MRI was performed with a 1.5 Tesla Siemens Magnetom Avanto B15 MR system (Siemens Healthcare, Erlangen, Germany) with the patient in the supine position. Body coil was used for imaging of the thoracic and lumbar regions while head coil and neck coil were used for the cervical region and brain imaging.
Imaging was done using FOV of 200–250 mm in infants and children and of 250–350 mm in older patients. First, T1-weighted (T1WI), T2-weighted (T2WI) and T2-weighted fat suppressed sequences were taken in the sagittal plane. Then, T1WI and T2WI were taken in axial plane in affected spinal regions. T2WI coronal sequence was also taken in cases with spinal curvature abnormality and vertebral segmentation defects. The chosen slice thickness was 3–4 mm with slice interval of 0.5–1.0 mm. Turbo spin-echo sequences were used with following parameters. T1WI: TR/TE/Flip Angle = 550/9.4/90°. T2WI: TR/TE/Flip Angle = 4000/110/150°.
Statistical analysis
The descriptive statistical approach was used to describe findings using numbers, percentage, and arithmetic mean. Comparison between USG and MRI findings and correlation of these findings with operative findings were also done using numbers and percentage.
Results
The study was carried out on 38 patients with SDs for 1 year extending from July 2014 to June 2015. The results and observations were as follows.
Demographic profile and clinical presentation
The age of the study group ranged from 2 days to 16 years. 84.21% patients presented before the age of 10 years and the neonatal period was the most common presenting age group accounting for 39.47% of total cases. 60.53% patients were female and 39.47% were male. Thus, females outnumbered males with a male to female ratio of 0.66.
The most common clinical finding at presentation was midline back swelling which was present in 23 (60.53%) patients. The next common finding was urinary incontinence in 18 (47.37%) patients which was followed by skin dimple in back in 11 (28.95%), fecal incontinence in 8 (21.05%), hair tuft in 1 (3.33%), and dermal sinus in 1 (3.33%).
Spinal segments involved
Lumbosacral spine was the most commonly involved spinal segment found in 20 (52.63%) patients. The next common region was sacrococcygeal, involved in 13 (34.21%) patients. The cervical and dorsal regions were involved in 4 (10.52%) and 1 (2.63%) patients, respectively.
Ultrasonography evaluation of spine
USG evaluation of spine was done in 29 cases where the ultrasonic acoustic window was available for adequate evaluation and following findings were appreciated. The most common finding on spinal USG was tethered cord, seen in 23 (79.31%) patients. The next common finding was syrinx in 18 (62.06%) followed by MMC in 14 (48.27%) and LipoMMC seen in 8 (27.58%) patients.
Type of spinal dysraphism and various spinal anomalies
OSD was found in 14 (36.84%) patients and CSD was seen in 24 (63.16%) patients. Of 24 patients with CSD, 10 (26.32%) patients had subcutaneous mass and 14 (36.84%) patients were not having subcutaneous mass. In open type of spinal anomaly, we found only MMC, which was also the most common anomaly overall, accounting for 14 (36.84%) cases [Graph 1]. The second most common anomaly was LipoMMC which was also the most common in closed type, seen in 9 (23.68%).
Graph 1.
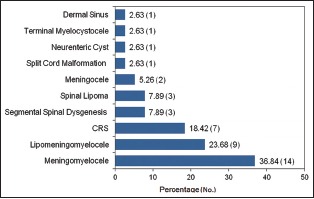
Summary of various spinal anomalies in this study
Caudal regression syndrome (CRS) was the third most common anomaly found in 7 (18.42%) cases.
Other anomalies associated with primary malformation
Vertebral anomalies were the most common associated abnormality found in 29 (76.32%) cases, of which spina bifida was the most common [Graph 2].
Graph 2.
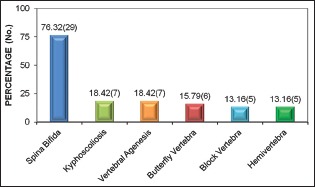
Vertebral anomalies associated with primary dysraphic lesion
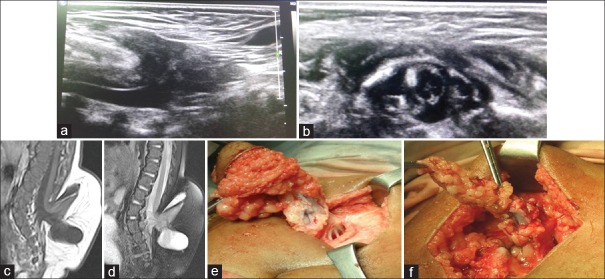
Other associated anomalies we encountered were tethered cord in 26 (68.42%) cases, syrinx in 21 (55.26%) cases, and ACM II in 13 (34.21%) cases. MMC was the most common dysraphic anomaly causing tethered cord and syrinx accounting for 14 out of 26 (53.84%) cases of tethered cord and 11 out of 21 (52.38%) cases of the syrinx. All 13 cases of ACM II were seen in association with MMC [Figure 1].
Figure 1.
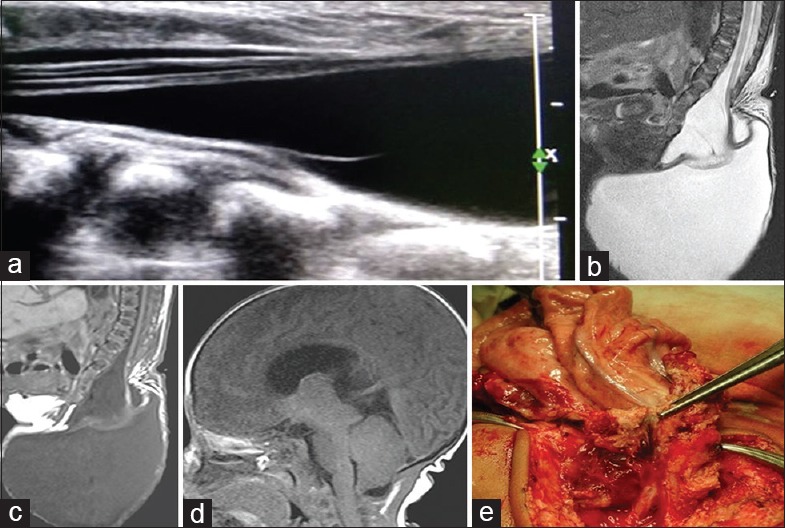
(a) Gray-scale spinal ultrasonography shows herniation of terminal cord and cerebrospinal fluid out of spinal canal with tethering of cord and focal syrinx. (b) Sagittal T2-weighted of L-S spine shows posterior elements defect in the sacral region through which there is herniation of large cerebrospinal fluid intensity cystic lesion in the sacrococcygeal region causing tethering of cord and mild syrinx formation. (c) Sagittal T1-weighted of L-S spine show findings similar to T2-weighted. No evidence of lipomatous tissue is noted. (d) Sagittal T1-weighted of brain shows caudal herniation of cerebellar vermis and brainstem, slit-like fourth ventricle, tectal beaking, and large massa intermedia, suggesting Chiari II malformation. (e) Peroperative photograph shows inner surface of the meningomyelocele sac attached to neural placode
Comparison between ultrasonography and magnetic resonance imaging findings
Both spinal USG and MRI were performed in 29 out of 38 cases. We could identify and diagnose primary anomaly on USG in 23 of 25 (92%) cases, and in MRI, we could diagnose primary anomaly in 25 cases (4 cases were having isolated vertebral anomalies or sacrococcygeal agenesis were not counted here as USG is not ideal modality for their diagnosis) [Graph 3a]. For identifying other abnormalities associated with spinal dysraphism such as tethered cord, syrinx, and split cord, USG was inferior to MRI [Graph 3b].
Graph 3.
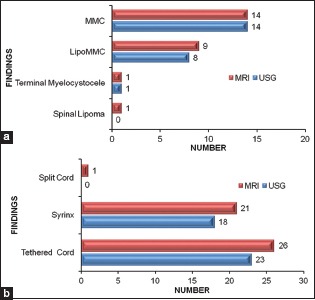
Comparison of ultrasound and magnetic resonance imaging findings for (a) primary anomaly, (b) associated abnormalities
We also determined agreement between findings of these two modalities as full when both showed all similar findings and partial when spinal USG was not able to show all the findings which were clearly recognized on MRI. 23 out of 29 (79.31%) cases showed full agreement between spinal USG and MRI examinations and 6 out of 29 (20.69%) showed partial agreement between spinal USG and MRI examinations. In these six cases, spinal USG missed tethered cord and syrinx in three cases, small lipomatous component in one case of LipoMMC, one case of intradural lipoma, and one case of split cord associated with MMC.
Correlation of imaging findings with operative findings in operated cases
We correlated spinal USG and MRI findings with operative findings in 23 cases who underwent operative management. MRI findings showed full correlation with operative findings in 23 out of 23 (100%) cases while USG findings correlated with operative findings in 21 out of 23 cases (91.30%) [Graph 4].
Graph 4.
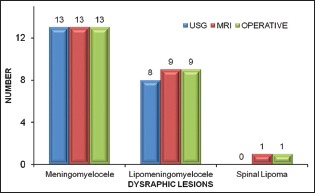
Correlation of imaging findings with operative findings in operated cases
Discussion
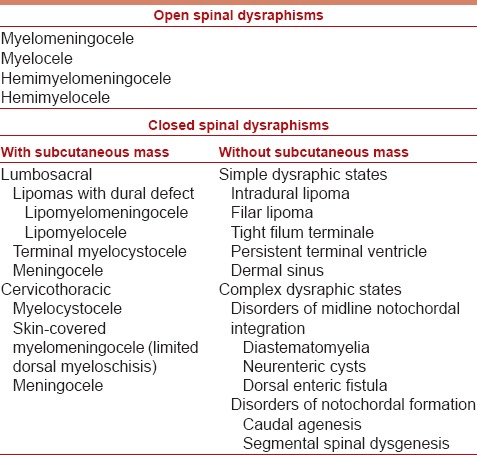
SD includes a spectrum of congenital spinal anomalies caused by the incomplete or abnormal closure of the neural tube during early embryogenesis. Their classification relies on the correlation of radiological features with a specific derangement in the normal developmental cascade.[7] Broadly, they can be divided into two groups: (1) Open type, in which there is exposure of abnormal nervous tissues through a skin defect, and (2) closed type, in which there is continuous skin coverage to the underlying malformation.[3] Closed group is further divided into two subsets based on whether a subcutaneous mass is present in the low back [Table 1].
Table 1.
Clinical-neuroradiological classification of spinal dysraphisms
Literature showed SDs present predominantly in the first decade of life and are more common in females.[3,4] Of 38 patients we studied, the youngest patient was of 2 days and the oldest patient was 16 years old. We observed slight female predominance with a male:female ratio of 0.66. SDs most commonly involve the lumbosacral spinal segment.[5,6] We observed similar predilection for lumbosacral region which was involved in 20 out of 38 (52.63%) cases.
Closed types of dysraphisms are more prevalent than open type accounting for 64.2% and 35.8% cases, respectively.[7] We found a similar result with closed anomalies accounted for 24 out of 38 (63.16%) cases and open for 14 out of 38 (36.84%) cases. MMC was the single most common dysraphic lesion in our study which matched with findings of previous studies.[8,9]
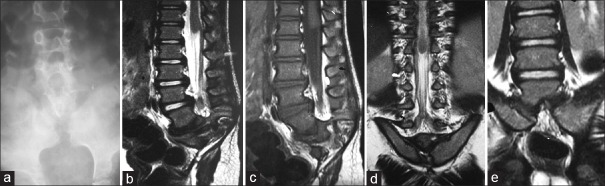
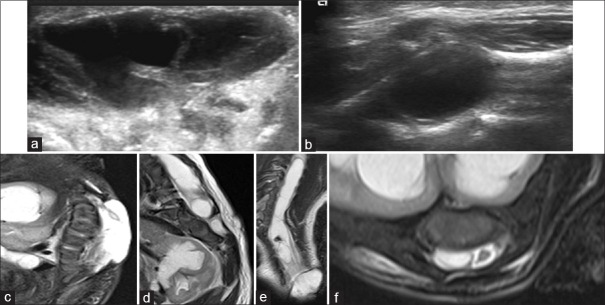
In closed group, we found LipoMMC [Figure 2a–f] as the most common anomaly accounted for 9 out of 38 (23.68%) cases followed by CRS [Figure 3a–e] in 7 out of 38 (18.42%) cases. The prevalence of CRS we found was higher compared to other studies.[3,8] This might be due to a higher prevalence of CRS in our region and detailed studies on CRS in future will be of great help in this regard.
Figure 2.
(a) Sagittal gray scale ultrasonography image shows herniation of neural placode and cerebrospinal fluid through posterior element dysraphism into subcutaneous fatty tissue. (b) Axial gray scale ultrasonography image shows lipomatous component attached to dorsolateral aspect of the spinal cord. (c) Sagittal T1-weighted shows a defect in posterior elements at lumbosacral junction with the expansion of subarachnoid space leading to herniation of neural placode and large T1 hyperintense lipoma attached to the neural plaque. (d) Sagittal T2-weighted fat suppressed image shows defect in posterior elements at lumbosacral junction with the expansion of subarachnoid space leading to herniation of neural placode. Lipoma attached to neural plaque showed signal suppression. (e) Peroperative image shows subcutaneous lipoma. (f) Peroperative images show neural placode to which lipomatous tissue is attached
Figure 3.
(a) Plain anteroposterior radiograph of lumbosacral spine shows a complete absence of sacrum and both iliac bones are medially oriented. (b) Sagittal T2-weighted shows complete sacrococcygeal agenesis, last visualized vertebrae is L5 and club-shaped or bulbous conus (arrow) terminating at L1 vertebral level without evidence of tethering or syrinx. (c) Sagittal T1-weighted shows gradual narrowing of the terminal thecal sac with mildly increased epidural fat in the lower lumbar region. No appreciable intradural lipoma was seen. (d) Coronal T2-weighted shows club-shaped or bulbous conus and more medially oriented posterior iliac bones reaching up to midline. (e) Coronal T2-weighted shows articulation between medially oriented iliac bones and L5
Split cord malformations (SCMs) are rare forms of CSD where the spinal cord is divided over a portion of its length into two equal or unequal halves.[10] SCMs are of two types; type I [Figure 4] consists of two hemicords, each contained within its own dural sheath and separated by a median bony spur, and type II consists of two hemicord housed in a single dural tube separated by a fibrous median septum.[11]
Figure 4.
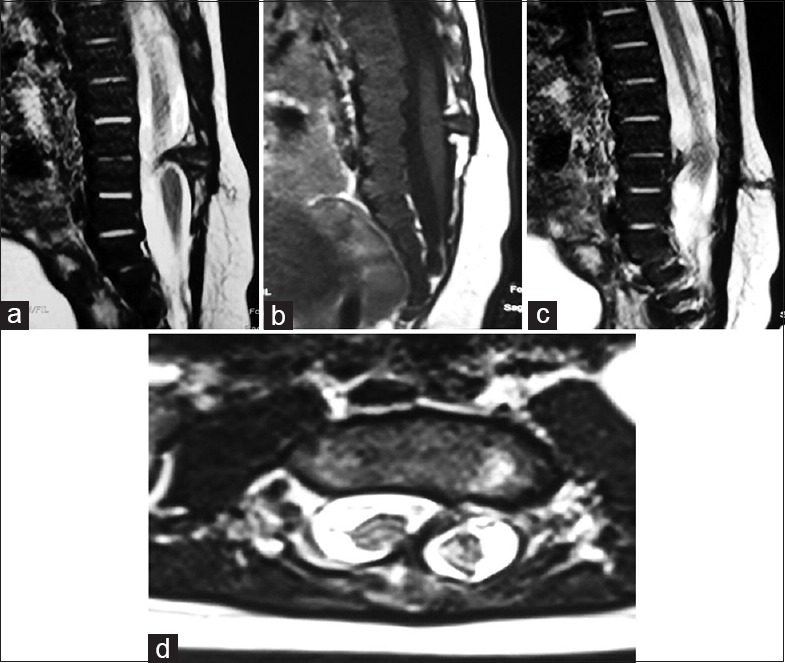
(a) Sagittal T2-weighted image shows anteroposteriorly oriented hypointense bony bar at L3/4 vertebral level. (b) Sagittal T1-weighted shows low-lying and tethered cord extending up to S3/4 vertebral level. (c) Sagittal T2-weighted shows focal syrinx in lower cord behind L1 and L2 vertebrae and hypointense sinus tract in subcutaneous fat extending from skin to spinous process at L4 vertebral level. (d) Axial T2-weighted shows two spinal cord separated by anteroposteriorly oriented hypointense spur
SCM can occur as isolated anomaly or it can also occur in association with MMC. Kumar et al.[12] labeled this combined pathology “complex spina bifida,” and suggested a minor modification to Pang's classification to accommodate the pure/combined anomalies together. Mahapatra and Gupta[13] further proposed a new subclassification for type I SCMs, wherein they divided type I SCMs into four types and found it to have a significant prognostic value as chances of injury to cord during surgery appear to be higher in some particular type which has a bearing on subsequent prognosis. According to this subclassification, type I SCM has been subdivided based on the location of the bony spur as follows:
Type Ia: Bony spur in the center with equally duplicated cord above and below the spur
Type Ib: Bony spur at the superior pole with no space above and a large duplicated cord lower down
Type Ic: Bony spur at the lower pole with a large duplicated cord above
Type Id: Bony spur straddling the bifurcation with no space above or below the spur.
We found 1 case of complex spina bifida, in which type I SCM was associated with MMC [Figure 5]. Further, in this case, there was a splitting of cord from upper dorsal level till termination with the bony bar at lower dorsal level. Hence, according to above-mentioned modified classification by Mahapatra and Gupta,[13] it fits into type Ia SCM.
Figure 5.
(a) Axial gray scale ultrasonography of swelling shows heteroechoic cystic lesion with linear echogenic strands within. (b) Axial ultrasonography image of lumbar spine shows syringomyelia. (c) Sagittal T2-weighted fat suppressed image shows lumbosacral meningomyelocele. Visualized kidney shows hydronephrosis. (d) Sagittal T2-weighted fat suppressed image shows oblique hypointense anteroposterior bar in spinal canal at the lower dorsal level and multilevel vertebral segmentation anomalies. Dilate upper pole moiety (collecting system) of the kidney is also visualized.(e) Coronal T2-weighted shows lumbosacral meningomyelocele, two spinal cords from about mid-dorsal level till termination with a syrinx in right-sided hemicord and obliquely oriented T2 hypointense spur. (f) Axial T2-weighted fat suppressed image shows two spinal cords with syrinx in right-sided hemicord
Vertebral anomalies were the most common of other anomalies which were associated with primary malformation in our study which correlated well with previous studies.[9,14] Chiari II malformation is a congenital hindbrain anomaly characterized by a small posterior fossa with caudal displacement of the cerebellar vermis and brainstem with slit like fourth ventricle, tectal beaking, large massa intermedia, medullary kink and medullary spur. It nearly, always occurs in association with OSD, and according to some authors, there are 100 associations between OSDs and the Chiari II malformation.[7,15] We found Chiari II malformation in 13 out of 38 (34.21%) cases and all cases were seen in association with MMC.
Role of spinal ultrasonography and magnetic resonance imaging in evaluation of spinal dysraphisms
Spinal ultrasound is becoming increasingly accepted as a first-line screening test in neonates suspected of SD.[16] Motion artifacts, which can considerably reduce the quality of MRI in young children, play a minor role during the real-time US.[17] For diagnosing main spinal anomalies in infants, diagnostic value of spinal USG is nearly similar to MRI.[5]
We found a good correlation between spinal USG and MRI findings for detecting primary dysraphic lesions such as MMC and LipoMMC with 92% of USG diagnosis exactly matched with that of MRI. However, spinal USG was not able to detect associated findings such as tethered cord, syrinx, split cord, exact extension of lipomatous tissue, and dermal sinus tract in 20.69% of USG examinations in our study.
Spinal USG has less sensitivity compared to MRI in detecting other findings associated with primary anomaly, and for detecting closed type of spinal anomalies, its sensitivity is significantly less in comparison to MRI.[18,19,20] Various vertebral anomalies and kyphoscoliotic deformity are commonly associated with these lesions which cannot be detected on USG. MRI is also superior in identifying exact extent of anomaly such as intraspinal extension of lipomatous tissue, level, extent and type of split cord, extent of vertebral agenesis, and shape and level of conus termination in case of CRS. Thus, MRI identifies all concurrent abnormalities, gives a complete diagnosis, and helps in planning better management.
Conclusion
SDs are one of the most common congenital disorders associated with significant morbidity and mortality. They result from defects in the early embryonic stages between 2nd and 6th gestational weeks. Knowledge of normal embryology is essential for understanding the pathogenesis and neuroradiology of these anomalies. We believe that spinal USG is the initial investigation for suspected cases of SDs, especially in infants as it is easily available, easy to perform without sedation, less costly than MRI, and has good sensitivity in diagnosing primary anomaly. However, MRI is the investigation of choice for evaluation of these complex anomalies and should be performed whenever possible because of following reasons:
A significant portion of patients present with concurrent anomalies that need to be addressed simultaneously to avoid repeat surgeries
MRI identifies all anomalies simultaneously and gives a complete diagnosis as well as additional anatomical details which help in better management and prognostication of these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rossi A, Cama A, Piatelli G, Ravegnani M, Biancheri R, Tortori-Donati P. Spinal dysraphism: MR imaging rationale. J Neuroradiol. 2004;31:3–24. doi: 10.1016/s0150-9861(04)96875-7. [DOI] [PubMed] [Google Scholar]
- 2.Venkataramana NK. Spinal dysraphism. J Pediatr Neurosci. 2011;6(Suppl 1):S31–40. doi: 10.4103/1817-1745.85707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravi N, Ramesh V, Naveen KG, Nagaraj BR, Adarsh KM, Manjappa HB, et al. Role of MRI in the evaluation of spinal dysraphism. SSRG Int J Med Sci. 2014;2:31–44. [Google Scholar]
- 4.Sarica K, Erbagci A, Yagci F, Yurtseven C, Buyukbebeci O, Karakurum G. Multidisciplinary evaluation of occult spinal dysraphism in 47 children. Scand J Urol Nephrol. 2003;37:329–34. doi: 10.1080/00365590310004725. [DOI] [PubMed] [Google Scholar]
- 5.Zakaria YM, Hegab SE, El-Fawal SK, Orz YI. Diagnostic value of spinal ultrasound: A comparative study with magnetic resonance imaging in infants with spinal dysraphism. Bull Alex Fac Med. 2005;4:200. [Google Scholar]
- 6.Kumar R, Singh SN. Spinal dysraphism: Trends in Northern India. Pediatr Neurosurg. 2003;38:133–45. doi: 10.1159/000068819. [DOI] [PubMed] [Google Scholar]
- 7.Tortori-Donati P, Rossi A, Cama A. Spinal dysraphism: A review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology. 2000;42:471–91. doi: 10.1007/s002340000325. [DOI] [PubMed] [Google Scholar]
- 8.Sadiq S, Faiq SM, Idrees MK. Lumbosacral dysraphism as cause of neurogenic bladder: Magnetic resonance imaging based study from SIUT Pakistan. J Pak Med Assoc. 2015;65:501–5. [PubMed] [Google Scholar]
- 9.Nishtar T, Elahi A. To determine the frequency of accuracy of MRI in diagnosis of rare disorder of spinal dysraphism. J Med Sci. 2011;19:195–9. [Google Scholar]
- 10.Kumar R, Singh V, Singh SN. Split cord malformation in children undergoing neurological intervention in India: A descriptive study. J Pediatr Neurol. 2004;2:21–7. [Google Scholar]
- 11.Pang D, Dias MS, Ahab-Barmada M. Split cord malformation: Part I: A unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992;31:451–80. doi: 10.1227/00006123-199209000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Bansal KK, Chhabra DK. Occurrence of split cord malformation in meningomyelocele: Complex spina bifida. Pediatr Neurosurg. 2002;36:119–27. doi: 10.1159/000048366. [DOI] [PubMed] [Google Scholar]
- 13.Mahapatra AK, Gupta DK. Split cord malformations: A clinical study of 254 patients and a proposal for a new clinico-imaging classifiation. J Neurosurg Pediatr. 2005;103:531–6. doi: 10.3171/ped.2005.103.6.0531. [DOI] [PubMed] [Google Scholar]
- 14.Ramacharya, Desai KS. Spinal dysraphism: MRI evaluation. Int J Res Med Sci. 2015;3:1937–41. [Google Scholar]
- 15.Hashim AS, Ahmed S, Jooma R. Management of myelomeningocele. J Surg Pak (Int) 2008;13:7–11. [Google Scholar]
- 16.Dick EA, Patel K, Owens CM, De Bruyn R. Spinal ultrasound in infants. Br J Radiol. 2002;75:384–92. doi: 10.1259/bjr.75.892.750384. [DOI] [PubMed] [Google Scholar]
- 17.Dick EA, de Bruyn R, Patel K, Owens CM. Spinal ultrasound in cloacal exstrophy. Clin Radiol. 2001;56:289–94. doi: 10.1053/crad.2000.0648. [DOI] [PubMed] [Google Scholar]
- 18.Chern JJ, Aksut B, Kirkman JL, Shoja MM, Tubbs RS, Royal SA, et al. The accuracy of abnormal lumbar sonography findings in detecting occult spinal dysraphism: A comparison with magnetic resonance imaging. J Neurosurg Pediatr. 2012;10:150–3. doi: 10.3171/2012.5.PEDS11564. [DOI] [PubMed] [Google Scholar]
- 19.Kim SM, Chang HK, Lee MJ, Shim KW, Oh JT, Kim DS, et al. Spinal dysraphism with anorectal malformation: Lumbosacral magnetic resonance imaging evaluation of 120 patients. J Pediatr Surg. 2010;45:769–76. doi: 10.1016/j.jpedsurg.2009.10.094. [DOI] [PubMed] [Google Scholar]
- 20.Drolet BA, Chamlin SL, Garzon MC, Adams D, Baselga E, Haggstrom AN, et al. Prospective study of spinal anomalies in children with infantile hemangiomas of the lumbosacral skin. J Pediatr. 2010;157:789–94. doi: 10.1016/j.jpeds.2010.07.054. [DOI] [PubMed] [Google Scholar]