Abstract
Objective
To identify associations between second-trimester serum inflammatory biomarkers and preterm birth among obese women.
Methods
In this nested case-control study, we compared 65 serum inflammatory biomarkers in obese women whose pregnancies resulted in early spontaneous preterm birth (< 32 weeks gestation, n=34) to obese women whose pregnancies resulted in term birth (n=34). These women were selected from a larger population-based California cohort. Random forest and classification and regression tree techniques were employed to identify biomarkers of importance, and adjusted odds ratios (aORs) and 95% confidence intervals (CI) were estimated using logistic regression.
Results
Random forest and classification and regression tree techniques found that soluble vascular endothelial growth factor receptor-3 (sVEGFR3), soluble interleukin-2 receptor alpha-chain (sIL-2RA), and soluble tumor necrosis factor receptor 1 (sTNFR1) were related to preterm birth. Using multivariable logistic regression to compare preterm cases and term controls, decreased serum levels of sVEGFR3 and increased serum levels of sIL-2RA and sTNFR1 were associated with increased risk of preterm birth among obese women, aOR 3.2 (95% CI 1.0–9.9), aOR 2.8 (95% CI 0.9–9.0), and aOR 4.1 (95% CI 1.2 – 14.1), respectively.
Conclusions
In this pilot study, we identified three serum biomarkers indicative of inflammation to be associated with spontaneous preterm birth among obese women: sVEGFR3, sIL-2RA, and sTNFR1.
Keywords: Inflammation, Preterm Birth, Inflammatory Markers, Obesity, vascular endothelial growth factor receptor-3, soluble interleukin-2 receptor alpha-chain, soluble tumor necrosis factor receptor I, vascular endothelial growth factor
INTRODUCTION
Globally, 15 million premature infants are born each year, with the highest rates in Africa and North America [1]. Thirteen percent of infants in the United States are born < 37 weeks gestation [2], the medical cost of which exceeds $26 billion per year [3]. Preterm birth (PTB) is a major cause of morbidity and mortality, and it accounts for approximately three fourths of all neonatal deaths [4, 5].
PTB is a complex phenomenon that is not well understood. Any disease process that induces myometrial contractility, cervical dilatation, and rupture of amniotic membranes—known as the “common pathway” of labor—prior to 37 weeks gestation can lead to spontaneous PTB [6]. Inflammatory cytokines and chemokines have been implicated in this “common pathway” and may mediate the association between certain disease states and PTB, such as intra-amniotic infection [7–11].
We recently reported an association between women’s pre-pregnancy obesity and early (<32 weeks gestation) PTB [12], which was consistent with earlier reports [13–14]. Since obesity itself is associated with chronic inflammation [15–17], we hypothesized that inflammatory processes may underlie the association between PTB and obesity.
In this nested case-control study, we examined biomarkers of inflammation in second-trimester serum samples, collected as part of routine screening for aneuoploidies and neural tube defects, in obese women who delivered prior to 32 weeks gestation compared to obese women who delivered at term.
METHODS
In this nested case-control study, we selected a subset of spontaneous PTB cases (< 32 weeks gestation, n=34) and term controls (≥ 37 weeks gestation, n=34) for comparison. All 68 case and control women were obese at the beginning of pregnancy, defined as body mass index (BMI) ≥ 30 kg/m2, based on pre-pregnancy weight divided by height squared. These 68 obese women were selected from a larger cohort of 1000 women, who all had first and second trimester prenatal screening for aneuoploidies and neural tube defects through the California Genetic Disease Screening Program from July 2009 through December 2010. Details of the larger cohort have been described previously [18–25].
All collected samples were obtained at 15–20 weeks gestation and were stored by the California Biobank Program. Ultrasound dating was performed as part of routine prenatal screening. Demographic and obstetric information was collected from a linked hospital discharge birth cohort database maintained by the California Office of Statewide Health Planning and Development (OSHPD) that includes linked information from vital records and hospital discharge records. From this dataset, the study included maternal pre-pregnancy BMI, age, race/ethnicity, education level, smoking, payer type, gestational age at delivery, parity, history of previous preterm birth, preexisting diabetes, gestational diabetes, preexisting hypertension, gestational hypertension, premature rupture of membranes, smoking, payer type, infant sex, gestational age at blood draw, and gestational age at delivery. Coding of diabetes, gestational diabetes, and hypertension was based on the International Classification of Diseases four digit codes contained in the cohort file.
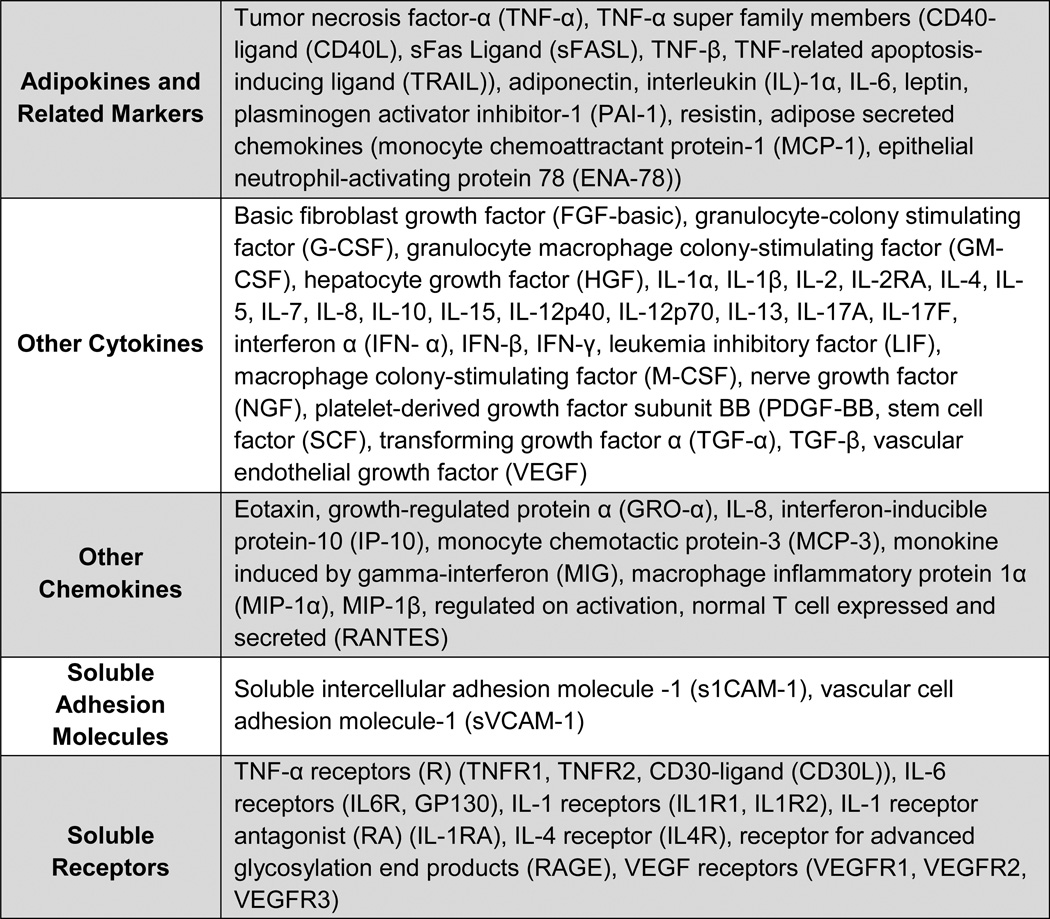
Inflammatory biomarkers were measured in second-trimester serum samples. These samples were obtained from the California Biobank, where samples are stored in 1-mL tubes at −80°C until thawed for testing. In this study, all testing was performed by the Human Immune Monitoring Center (HIMC) at Stanford University. Serum testing of cytokines, chemokines, and soluble adhesion molecules was performed using a human 51-plex kit (Affymetrix Inc., Santa Clara, CA) (Figure 1, rows 1–4). Human soluble receptors were measured using a Millipore high sensitivity 14-plex kit (HSCRMAG32KPX14) (Billerica, MA) (Figure 1, row 5). Adiponectin was measured using a Millipore high sensitivity kit (HADK1MAG-61K) (Billerica, MA). The complete list of biomarkers is shown in Figure 1. All biomarkers were read per manufacturer recommendations using a Luminex 200 instrument (Austin, TX). Luminex lab protocols at HIMC have been described previously [26]. Median fluorescence intensity (MFI) values were reported for all markers using Masterplex software (Hitashi Solutions, San Bruno, CA). All analyses relied on the MFI average of two aliquots tested on the same plate for each case and control. All inter-assay coefficients were <15% across all markers and all intra-assay coefficients were <10%.
Figure 1.
List of serum biomarkers.
Statistical Analysis
Cases and controls were compared on all 65 analytes. Random forest, along with classification and regression tree (CART), techniques were employed to evaluate whether analytes differed between cases and controls. Random forest is a tree-based machine-learning algorithm that determines variable importance through a series of permutations and randomized node-optimizations. Random forest allows for examination of multiple factors simultaneously and accounts for interactions between those factors and non-linear associations with outcomes [27–28]. Party package in R software (Version 3.1.1) was used to run random forest. CART is also a machine-learning technique that we used (http://web.stanford.edu/~yesavage/ROC.html). Similar to random forest, CART identifies variables of relative importance in their contribution to the outcome of interest using recursive partitioning [29]. For CART, we did not perform replication owing to small sample sizes. For Random Forest to provide an interpretation of the best predictors, we calculated a variable importance measure for each potential predictor variable, using the “varimp ” function in the party package in R software, using the metric Mean Decrease Accuracy (MDA) [28]. To verify stability of measures, we did Jackknife sampling 100 times (randomly sampled 90% of the original data for 100 times) and calculated median and median absolute deviation of MDA out of 100 in permuted data, then compared the two ranking lists – MDA from original dataset, and median of MDA out of 100 permutated data.
Generalized additive regression models (GAM) with polynomial spline estimation were examined for each analyte to confirm data contour and suggested cutoffs values from CART outputs. Multivariable logistic regression was performed and adjusted odds ratios (aOR) and 95% confidence intervals (CI) were estimated for analytes identified as differing between cases and controls by random forest and CART, controlling for potential covariates (SAS 9.4, SAS Institute, Cary, NC).
Methods and protocols for the study were approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California and the Institutional Review Board of Stanford University.
RESULTS
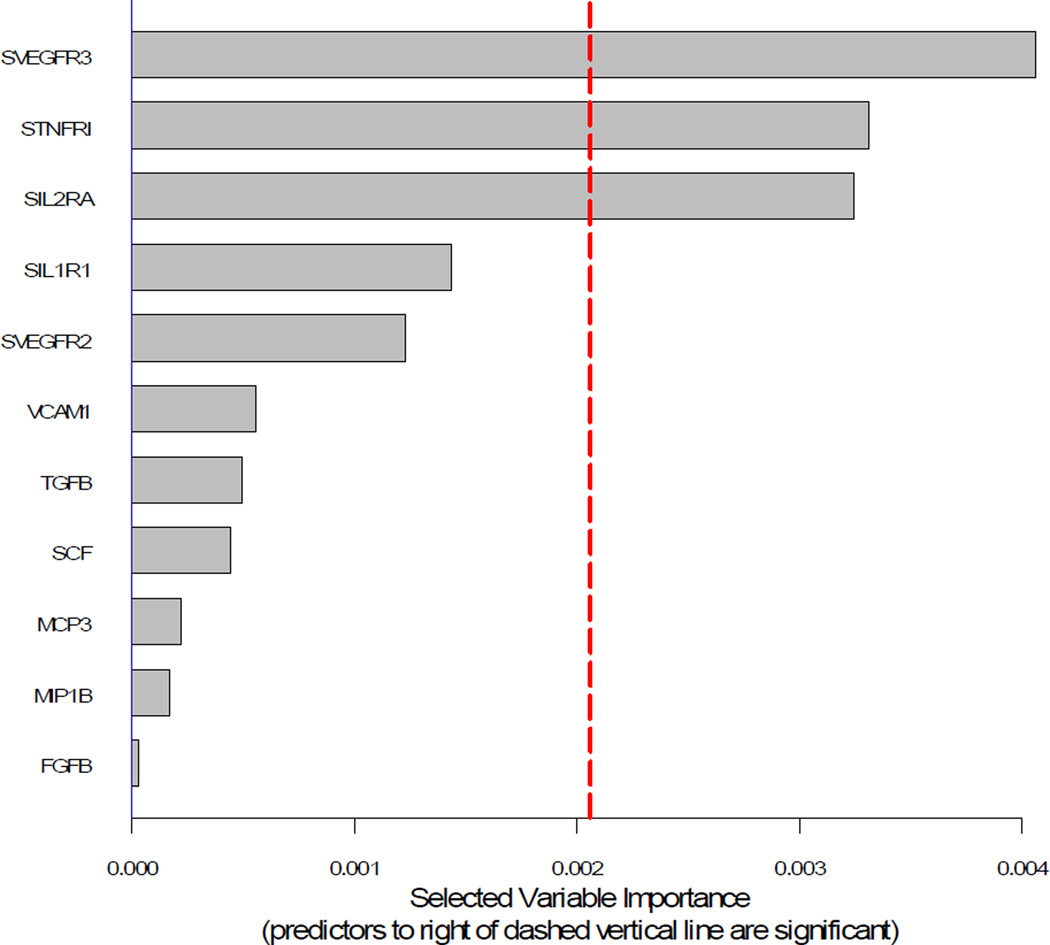
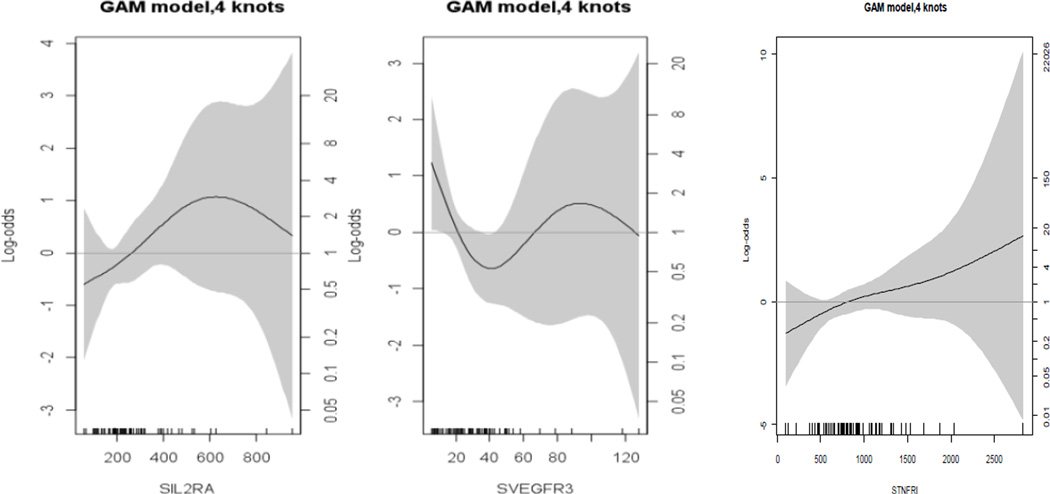
Demographic characteristics for PTB cases and term controls are shown in Table 1. Comparisons of the analytes between the two groups using means are shown in Table 2. There were no statistically significant differences in means (i.e., p < 0.01) between PTB cases and term controls. Random forest ranking plot identified three analytes of importance in sorting PTB cases from term controls: soluble vascular endothelial growth factor receptor 3 (sVEGFR3), soluble tumor necrosis factor receptor 1 (sTNFR1), and soluble interleukin-2 receptor alpha-chain (sIL-2RA) (Figure 2). CART decision tree analysis also identified sVEGFR3 and sIL-2RA as the most important markers. Suggested cutoff values were confirmed by spline estimation (Figure 3). The median value among term controls was used as the cutoff value for sTNFR1.
Table 1.
Demographic characteristics of the preterm cases and term controls.
| Variables | Preterm/obese (n=34) 1 |
Term/obese (n=34) 1 |
|
|---|---|---|---|
| Age (years) | |||
| <18 | 1 (2.9) | 0 | |
| 18–34 | 27 (79.4) | 24 (70.6) | |
| ≥35 | 6 (17.7) | 10 (29.4) | |
| Race/Ethnicity | |||
| Non-Hispanic White | 11 (32.4) | 11 (32.4) | |
| Hispanic | 21 (61.8) | 21 (61.8) | |
| Black | 1 (2.9) | 1 (2.9) | |
| Asian/Other | 1 (2.9) | 1 (2.9) | |
| Education | |||
| Some high school | 6 (17.7) | 7 (20.6) | |
| High school | 13 (38.2) | 9 (26.5) | |
| Some college | 7 (20.6) | 11 (32.4) | |
| ≥4 year college degree | 7 (20.6) | 5 (14.7) | |
| Prepregnancy BMI (kg/m2) |
|||
| Obese I (30 – 34.9) | 23 (67.7) | 17 (50.0) | |
| Obese II (35–39.9) | 5 (14.7) | 9 (26.5) | |
| Obese III (≥40) | 6 (17.7) | 8 (23.5) | |
| Parity | |||
| 1 | 18 (52.9) | 7 (20.6) | |
| ≥2 | 16 (47.1) | 27 (79.4) | |
| Previous preterm2 | |||
| No | 15 (93.8) | 27 (100.0) | |
| Yes | 1 (6.3) | 0 | |
| Any diabetes | |||
| No | 17 (50.0) | 29 (85.3) | |
| Yes | 17 (50.0) | 5 (14.7) | |
| Any hypertension | |||
| No | 24 (70.6) | 28 (82.4) | |
| Yes | 10 (29.4) | 6 (17.7) | |
| Any PROM3 | |||
| No | 19 (55.9) | 34 (100.0) | |
| Yes | 15 (44.1) | -- | |
| Any smoking | |||
| No | 32 (94.1) | 34 (100.0) | |
| Yes | 2 (5.9) | 0 | |
| Payer type for delivery | |||
| Medi-Cal | 22 (64.7) | 23 (67.7) | |
| Private | 10 (29.4) | 10 (29.4) | |
| Self-pay | 1 (2.9) | 0 | |
| Other government | 1 (2.9) | 1 (2.9) | |
| Infant sex | |||
| Male | 22 (64.7) | 15 (44.1) | |
| Female | 12 (35.3) | 19 (55.9) | |
Obesity is defined as body mass index (BMI) ≥ 30. Numbers may not add to 100% due to rounding or missing values
The denominator is those with parity ≥ 2
Premature rupture of membranes (PROM)
Table 2.
Comparisons of sample median fluorescence intensity (MFI) of biomarkers between the preterm cases and term controls using mean and standard deviation.
| Variable | Term/Obese | Preterm/Obese | p-value |
|---|---|---|---|
| ADIPON | 3216.6 (1776.9) | 3021.9 (1535.8) | 0.64 |
| SCD30 | 15.3 (25.5) | 10.8 (5.7) | 0.08 |
| SGP130 | 11082.5 (1326.0) | 11037.1 (1513.6) | 0.89 |
| sIL-1R1 | 38.6 (9.3) | 35.9 (9.8) | 0.26 |
| sIL-1RII | 579.3 (349.2) | 713.4 (393.6) | 0.13 |
| sIL-2RA | 242.3 (149.4) | 300.9 (180.0) | 0.13 |
| sIL-4R | 106.0 (98.7) | 112.5 (134.6) | 0.80 |
| sIL-6R | 12599.2 (2328.9) | 12576.3 (2193.6) | 0.97 |
| sRAGE | 43.0 (24.4) | 43.2 (24.9) | 0.99 |
| sTNFRI | 778.9 (370.2) | 997.1 (506.6) | 0.04 |
| sTNFRII | 5221.9 (1248.5) | 5252.7 (1797.2) | 0.93 |
| sVEGFR1 | 93.5 (39.4) | 109.2 (74.4) | 0.21 |
| sVEGFR2 | 482.6 (108.1) | 456.0 (110.3) | 0.36 |
| sVEGFR3 | 34.2 (22.4) | 29.0 (24.6) | 0.49 |
| LEPTIN | 8556.0 (2857.6) | 8681.0 (3521.9) | 0.85 |
| SCF | 290.2 (103.7) | 277.1 (73.2) | 0.46 |
| MIG | 409.2 (297.1) | 376.8 (100.1) | 0.33 |
| MIP1-α | 182.9 (93.9) | 215.7 (353.9) | 0.69 |
| MCP3 | 274.1 (93.0) | 286.6 (85.6) | 0.55 |
| PAI1 | 5357.7 (628.4) | 5387.9 (674.4) | 0.87 |
| SFASL | 397.7 (192.5) | 378.3 (161.5) | 0.60 |
| ENA78 | 321.7 (370.3) | 283.2 (321.8) | 0.47 |
| IL-1 β | 292.0 (139.7) | 383.1 (455.3) | 0.74 |
| IL-2 | 271.1 (112.5) | 282.0 (105.3) | 0.63 |
| IL-4 | 243.9 (94.9) | 239.8 (73.1) | 0.80 |
| IL-5 | 203.0 (98.4) | 201.8 (75.9) | 0.94 |
| IP10 | 264.5 (100.3) | 262.6 (68.1) | 0.97 |
| TGF-α | 216.7 (81.6) | 203.9 (70.4) | 0.46 |
| IL-6 | 126.2 (66.2) | 159.0 (224.2) | 0.32 |
| IL-7 | 367.6 (199.9) | 383.3 (164.5) | 0.65 |
| IL-8 | 2757.1 (2926.8) | 3143.6 (3502.0) | 0.64 |
| IL-10 | 239.6 (93.3) | 222.9 (58.7) | 0.30 |
| TGF-β | 147.6 (59.5) | 162.4 (70.3) | 0.26 |
| IFN--β | 120.3 (53.3) | 128.6 (70.9) | 0.57 |
| TNF-β | 362.3 (149.9) | 381.4 (150.6) | 0.56 |
| IL-12P40 | 792.2 (271.8) | 756.9 (175.4) | 0.51 |
| IL-12P70 | 188.3 (75.8) | 182.4 (53.9) | 0.65 |
| IL-13 | 104.2 (45.7) | 98.7 (38.7) | 0.53 |
| IL-17 | 174.9 (72.5) | 170.1 (58.6) | 0.74 |
| PDGF- BB |
766.8 (423.7) | 795.8 (434.2) | 0.73 |
| NGF | 71.4 (31.1) | 61.7 (16.7) | 0.03 |
| IL-17F | 160.0 (65.7) | 153.6 (62.8) | 0.80 |
| RANTES | 12801.9 (1410.6) | 12647.3 (1337.9) | 0.67 |
| IFN-γ | 249.3 (85.4) | 248.1 (71.8) | 0.94 |
| GMCSF | 196.4 (70.2) | 194.2 (64.0) | 0.88 |
| TNF-α | 155.2 (65.3) | 162.6 (96.7) | 0.59 |
| GCSF | 64.3 (16.6) | 61.1 (22.9) | 0.43 |
| MIP-1β | 226.8 (89.6) | 360.0 (874.8) | 0.19 |
| IFN-α | 105.5 (43.0) | 102.2 (41.6) | 0.73 |
| LIF | 180.4 (80.6) | 177.7 (68.4) | 0.87 |
| MCP1 | 724.1 (1244.6) | 581.1 (424.9) | 0.50 |
| EOTAXI | 226.0 (87.9) | 224.6 (60.5) | 0.93 |
| FGFB | 264.3 (105.3) | 262.7 (89.5) | 0.94 |
| VEGF | 134.8 (55.0) | 131.3 (53.3) | 0.79 |
| TRAIL | 185.6 (51.0) | 181.8 (49.0) | 0.76 |
| GROA | 252.2 (221.4) | 280.7 (422.0) | 0.65 |
| IL-1α | 196.0 (65.5) | 183.1 (44.1) | 0.29 |
| IL-1RA | 481.7 (734.2) | 442.6 (514.8) | 0.66 |
| IL-15 | 123.8 (44.2) | 120.6 (33.9) | 0.74 |
| ICAM1 | 7554.4 (4978.7) | 6831.3 (4118.0) | 0.42 |
| HGF | 535.2 (206.0) | 514.3 (203.1) | 0.61 |
| CD40L | 456.1 (485.7) | 363.7 (378.1) | 0.40 |
| RESIST | 4366.7 (1882.9) | 4517.1 (1934.6) | 0.74 |
| VCAM1 | 15052.7 (2302.5) | 13890.6 (3391.3) | 0.07 |
| MCSF | 270.1 (108.4) | 289.5 (128.8) | 0.45 |
Figure 2.
Random forest ranking plot.
Figure 3.
Generalized additive regression models (GAM) with polynomial spline estimation for sIL2RA, sVEGFR3, and sTNFR1.
Logistic regression was performed for sVEGFR3, sIL-2RA, and sTNFR1. Among study women (all of whom were obese), serum sVEGFR3 < 23 (vs. ≥ 23), sIL-2RA ≥ 271.5 (vs. < 271.5), and sTNFR1 ≥ 733.0 (vs. < 733.0) were associated with PTB with aOR 3.2 (95% CI 1.0–9.9), aOR 2.8 (95% CI 0.9–9.0), and aOR 4.1 (95% CI 1.2 – 14.1), respectively, controlling for diabetes, hypertension, and parity. Patients with combined risk across multiple analytes were at higher risk of PTB, including sVEGFR3 < 23 and sIL-2RA ≥ 271.5 (aOR 8.9, 95% CI 1.7–47.3), sVEGFR3 < 23 and sTNFR1 ≥ 733.0 (aOR 52.6, 95% CI 3.6 – 764.2), and sTNFR1 ≥ 733.0 and sIL-2RA ≥ 271.5 (aOR 7.3, 95% CI 1.5 – 35.2), controlling for diabetes, hypertension, and parity.
DISCUSSION
In this study, we identified three inflammatory biomarkers—sVEGFR3, sIL-2RA, and sTNFR1—as being associated with spontaneous PTB among obese women in their mid-pregnancy serum. These findings are generally consistent with the hypothesis that inflammation may mediate the relationship between obesity and PTB, which we have described previously (Shaw 2014).
The group of vascular endothelial growth factor (VEGF) molecules (including VEGF, VEGFR1, VEGFR2, and VEGFR3) are important mediators of angiogenesis and direct growth of blood and lymphatic vasculature [30–31], and may contribute to fetal development and placental function [32–35]. Pathways related to angiogenesis have also been implicated in the etiology of preeclampsia [36–40]. VEGFR3 is a tyrosine kinase receptor expressed in lymphatic vessels, and is activated by ligand VEGF-C, which promotes lymphangiogenesis [41–42]. Activation of this pathway has been associated with both inflammatory and anti-inflammatory processes [43–46].
The IL-2/IL-2RA (CD25) pathway is central to immune regulation [47]. Elevated levels of IL-2RA have been found in patients with infectious, inflammatory, and autoimmune diseases [48–53]. The TNF-α pathway is involved in systemic inflammation and has been implicated in both PTB and lipid metabolism [22, 54–58].
While a number of other studies have implicated other VEGF-related markers in PTB and preeclampsia, we know of no previous studies that have investigated the potential association between sVEGFR3, sIL-2RA, or sTNFR1 and PTB. However, there are biologically plausible hypotheses regarding these markers and PTB, although the differences in directionality between the three analytes and PTB risk remains puzzling. We believe that obesity may alter vascular growth and lymphangiogenesis of the fetal-placental unit, which may lead to failed tolerance and consequent inflammation. The signaling pathways related to maternal inflammation may impact placentation and increase risk of PTB. Our results are generally consistent with the “common pathway” theory of labor and PTB [6].
These observations should be interpreted with caution owing to the relatively small study sample size and the attendant multiple analyses conducted for this initial discovery approach. However, two different machine-learning analytic techniques, which are not affected by multiple testing, led us to focusing on the same two biomarkers.
Despite these limitations, we believe that these initial pilot findings are worthy of further pursuit. The interaction between obesity, PTB, and inflammatory biomarkers may lead to further understanding of the mechanism of PTB, and, potentially, to novel therapies targeted towards these underlying mechanisms.
Acknowledgments
Statement of Financial Support: Supported by NIH grants (RO1 HD-57192 and R01 HD-52953), the March of Dimes Prematurity Center at Stanford University School of Medicine, the Stanford Child Health Research Institute at Stanford University School of Medicine, Bill and Melinda Gates Millennium grants (OPP52256 and RSDP 5K12 HD-00849-23), and March of Dimes grants (6-FY11-261 and FY10-180).
Abbreviations
- PTB
Preterm birth
- BMI
body mass index
- HIMC
Human Immune Monitoring Center
- MFI
median fluorescence intensity
- CART
classification and regression tree
- OR
odds ratio
- CI
95% confidence interval
- OSHPD
California Office of Statewide Health Planning and Development
- sVEGFR3
vascular endothelial growth factor receptor-3
- sTNFRI
soluble tumor necrosis factor receptor I
- sIL2RA
soluble interleukin-2 receptor alpha-chain
- VEGF
vascular endothelial growth factor
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Kung HC, Mathews TJ, Hoyert DJ, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 3.Behrman RE, Butler AS, editors. National Research Council. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. p. 400. [PubMed] [Google Scholar]
- 4.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. NEJM. 1966;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 5.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. 2012;17:12–19. doi: 10.1016/j.siny.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon R, Furtunato SJ. Infection and the role of inflammation in preterm premature rupture of he membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21:467–478. doi: 10.1016/j.bpobgyn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2007;27:1037–1051. doi: 10.1016/j.placenta.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15s2:41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 12.Shaw GM, Wise PH, Mayo J, Carrmichael SL, Ley C, Lyell DJ, et al. Maternal prepregnancy body mass index and risk of spontaneous preterm birth. Paediatr Perinat Epidemiol. 2014;28:302–311. doi: 10.1111/ppe.12125. [DOI] [PubMed] [Google Scholar]
- 13.Djelantik AA, Kunst AE, van der Wal MF, Smit HA, Vrijkotte TG. Contribution of overweight and obesity to the occurrence of adverse pregnancy outcomes in a multi-ethnic cohort: population attributive fractions for Amsterdam. BJOG. 2012;119:283–290. doi: 10.1111/j.1471-0528.2011.03205.x. [DOI] [PubMed] [Google Scholar]
- 14.Wise LA, Palmer JR, Heffner LJ, Rosenberg L. Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiology. 2010;21:243–252. doi: 10.1097/EDE.0b013e3181cb61a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed-Ali V, Goodrick S, Bulmer K, et al. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277(6 Pt 1):E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 16.Roytblat L, Rachinsky M, Fisher A, et al. Raised interleukin-6 levels in obese patients. Obes Res. 2000;8(9):673–675. doi: 10.1038/oby.2000.86. [DOI] [PubMed] [Google Scholar]
- 17.Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 18.Jelliffe-Pawlowski LL, Walton-Haynes L, Currier RJ. Using second trimester ultrasound and maternal serum biomarker data to help detect congenital heart defects in pregnancies with positive triple-marker screening results. Am J Med Genet A. 2008;146A:2455–2467. doi: 10.1002/ajmg.a.32513. [DOI] [PubMed] [Google Scholar]
- 19.Jelliffe-Pawlowski LL, Walton-Haynes L, Currier RJ. Identification of second trimester screen positive pregnancies at increased risk for congenital heart defects. Prenat Diagn. 2009;29:570–577. doi: 10.1002/pd.2239. [DOI] [PubMed] [Google Scholar]
- 20.Jelliffe-Pawlowski LL, Baer RJ, Currier RJ. Second trimester serum predictors of preterm birth in a population-based sample of low-risk pregnancies. Prenat Diagn. 2010;30:727–733. doi: 10.1002/pd.2489. [DOI] [PubMed] [Google Scholar]
- 21.Jelliffe-Pawlowski LL, Shaw GM, Stevenson DK, Oehlert JW, Quaintance C, Santos AJ, et al. Risk of bronchopulmonary dysplasia by second-trimester maternal serum levels of alpha-fetoprotein, human chorionic gonadotropin, and unconjugated estriol. Pediatr Res. 2012;71:399–406. doi: 10.1038/pr.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jelliffe-Pawlowski LL, Ryckman KK, Bedell B, O'Brodovich HM, Gould JB, Lyell DJ, et al. Elevated mid-pregnancy tumor necrosis factor-α (TNF-α) and lipid patterns suggestive of hyperlipidemia in pregnancies resulting in early preterm birth. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.02.019. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumenfeld YJ, Baer RJ, Druzin ML, El-Sayed YY, Lyell DJ, Faucett AM, et al. Association between maternal characteristics, abnormal serum aneuploidy analytes, and placental abruption. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.03.027. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Baer RJ, Currier RJ, Norton ME, Flessel MC, Goldman S, Towner D, et al. Obstetric, perinatal, and fetal outcomes in pregnancies with false-positive integrated screening results. Obstet Gynecol. 2014;123:603–609. doi: 10.1097/AOG.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 25.Jelliffe-Pawlowski LL, Baer RJ, Currier RJ, Lyell DJ, Blumenfeld YJ, El-Sayed YY, et al. Early-Onset Severe Preeclampsia by First Trimester Pregnancy-Associated Plasma Protein A and Total Human Chorionic Gonadotropin. Am J Perinatol. 2014 doi: 10.1055/s-0034-1396697. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Stringer EA, Baker KS, Carroll IR, Montoya JG, Chu L, Maecker HT, et al. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. J Transl Med. 2013;11:93. doi: 10.1186/1479-5876-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breiman L. Random forests. Machine learning. 2001;45:5–32. [Google Scholar]
- 28.Strobl C, Malley J, Tutz G. An Introduction to Recursive Partitioning: Rational, Application, and Characteristics of Classification and Regression Trees, Bagging, and Random Forests. Psychological Methods. 2009;14:323–348. doi: 10.1037/a0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and regression trees. CRC press; 1984. [Google Scholar]
- 30.Carmieliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 31.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2003;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 32.Cheung CY. Vascular endothelial growth factor: possible role in fetal development and placental function. J Soc Gynecol Invest. 1997;4:169–177. [PubMed] [Google Scholar]
- 33.Kumazaki K, Nakayama M, Suehara N, Wada Y. Expression of Vascular Endothelial Growth Factor, Placental Growth Factor, and Their Receptors Flt-1 and KDR in Human Placenta Under Pathologic Conditions. Human Pathology. 2002;33:1069–1077. doi: 10.1053/hupa.2002.129420. [DOI] [PubMed] [Google Scholar]
- 34.Lash GE, Cartwright JE, Whitley GJ, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999;20:661–667. doi: 10.1053/plac.1999.0427. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular Endothelial Growth Factor Ligands and Receptors That Regulate Human Cytotrophoblast Survival Are Dysregulated in Severe Preeclampsia and Hemolysis, Elevated Liver Enzymes, and Low Platelets Syndrome. Am J Pathology. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–207. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 38.Kim YN, Lee DS, Jeong DH, Sung MS, Kim KT. The relationship of the level of circulating antiangiogenic factors to the clinical manifestations of preeclampsia. Prenat Diagn. 2009;29:464–470. doi: 10.1002/pd.2203. [DOI] [PubMed] [Google Scholar]
- 39.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. NEJM. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 40.Almawi WY, Saldanha FL, Mahmood NA, Al-Zaman I, Sater MS, Mustafa FE. Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum Reprod. 2013;28:2628–2635. doi: 10.1093/humrep/det308. [DOI] [PubMed] [Google Scholar]
- 41.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 42.Lohela M, Saaristo A, Veikkola T, Alitalo K. Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost. 2003;90:167–184. doi: 10.1160/TH03-04-0200. [DOI] [PubMed] [Google Scholar]
- 43.Alitalo AK, Proulx ST, Karaman S, Aebischer D, Martino S, Jost M, et al. VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis. Cancer Res. 2013;73:4212–4221. doi: 10.1158/0008-5472.CAN-12-4539. [DOI] [PubMed] [Google Scholar]
- 44.Hagura A, Asai J, Maryuama K, Takenaka H, Kinoshita S, Katoh N. The VEGF-C/VEGFR3 signaling pathway contributes to resolving chronic skin inflammation by activating lymphatic vessel function. J Dermatol Sci. 2014;73:135–141. doi: 10.1016/j.jdermsci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Jurisic G, Sundberg JP, Detmar M. Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm Bowel Dis. 2013;19:1983–1989. doi: 10.1097/MIB.0b013e31829292f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 47.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 48.Adachi K, Kumamoto T, Araki S. Interleukin-2 receptor levels indicating relapse in multiple sclerosis. Lancet. 1989;1:559–560. doi: 10.1016/s0140-6736(89)90103-7. [DOI] [PubMed] [Google Scholar]
- 49.Gallo P, Piccinno MG, Pagni S, Argentiero V, Giometto B, Bozza F, Tavolato B. Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and γ-IFN levels in serum and cerebrospinal fluid. J Neurol Sci. 1989;92:9–15. doi: 10.1016/0022-510x(89)90171-8. [DOI] [PubMed] [Google Scholar]
- 50.Giordano C, Galluzzo A, Marco A, Panto F, Amato MP, Caruso C, Bompiani GD. Increased soluble interleukin-2 receptor levels in the sera of type 1 diabetic patients. Diabetes Res. 1988;8:135–138. [PubMed] [Google Scholar]
- 51.Greenberg SJ, Marcon L, Hurwitz BJ, Waldmann TA, Nelson DL. Elevated levels of soluble interleukin-2 receptors in multiple sclerosis. NEJM. 1988;319:1019–1020. doi: 10.1056/NEJM198810133191517. [DOI] [PubMed] [Google Scholar]
- 52.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Makis AC, Galanakis E, Hatzimichael EC, Papadopoulou ZL, Siamopoulou A, Bourantas KL. Serum levels of soluble interleukin-2 receptor α (sIL-2Rα) as a predictor of outcome in brucellosis. J Infect. 2005;51:206–210. doi: 10.1016/j.jinf.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 55.Pearce BD, Grove J, Bonney EA, et al. Interrelationship of cytokines, hypothalamic-pituitary-adrenal axis hormones, and psycho-social variables in the prediction of preterm birth. Gynecol Obstet Invest. 2010;70:40–46. doi: 10.1159/000284949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coussons-Read ME, Lobel M, Carey JC, et al. The occurance of preterm delivery is linked to pregnancy-specifi cdistress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26:650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruan H, Hacohen N, Golub TR, Van PL, Lodish HF. Tumor necrosis factor-α suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-α is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Xun K, Chen L, Want Y. TNF-α, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27:407–416. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]