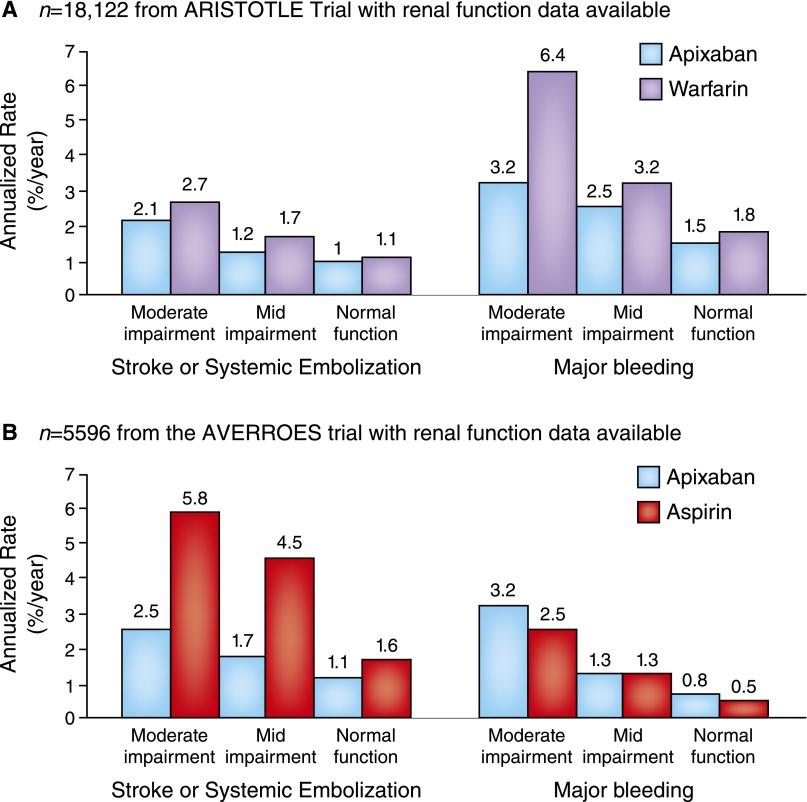
Figure 1.
Bleeding and stroke/systemic embolism by renal function group in novel oral anticoagulant pivotal trials. (A and B) Bleeding and stroke/systemic embolism by renal function in novel oral anticoagulant pivotal trials. For the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial according to baseline Cockcroft–Gault calculated creatinine clearance, there were 7518 patients (42%) with an eGFR of >80 ml/min, 7587 patients (42%) with an eGFR between >50 and 80 ml/min, and 3017 patients (15%) with an eGFR of ≤50 ml/min. For the Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) Trial, among 5596 patients, there were 1628 patients (29%) with an eGFR of >80 ml/min, 3084 patients (55%) with an eGFR between >50 and 80 ml/min, and 884 patients (16%) with an eGFR of ≤50 ml/min.