Abstract
Local adaptation is used as a criterion to select plant materials that will display high fitness in new environments. A large body of research has explored local adaptation in plants, however, to what extent findings can inform management decisions has not been formally evaluated. We assessed local adaptation literature for six key experimental methodologies that have the greatest effect on the application of research to selecting plant materials for natural resource management: experimental environment, response variables, maternal effects, intraspecific variation, selective agents, and spatial and temporal variability. We found that less than half of experiments used reciprocal transplants or natural field conditions, which are both informative for revegetation and restoration. Population growth rate was rarely (5%) assessed, and most studies measured only single generations (96%) and ran for less than a year. Emergence and establishment are limiting factors in successful revegetation and restoration, but the majority of studies measured later life‐history stages (66%). Additionally, most studies included limited replication at the population and habitat levels and tested response to single abiotic selective factors (66%). Local adaptation research should be cautiously applied to management; future research could use alternative methodologies to allow managers to directly apply findings.
Keywords: ecological experiments, experimental design, experimental methodology, lifetime fitness, local adaptation, plants
Introduction
Local adaptation is the process by which resident genotypes exhibit higher fitness in their home environment compared with nonlocal genotypes due to divergent selection as a consequence of variation in environment (Kawecki and Ebert 2004). Over the course of the 20th century, research on local adaptation has expanded from a primary focus on long‐term evolutionary processes, such as speciation (Jordan 1905), to a broader set of issues including rapid evolutionary processes and responses to changing environmental conditions (Barrett et al. 2008; Leger and Espeland 2010; Hoffmann and Sgro 2011).
Meanwhile, scientists and managers are increasingly using results of local adaptation research (LAR) to inform complex management decisions (Hufford and Mazer 2003), such as assisted migration for climate change mitigation (Vitt et al. 2010), and choice of native plant materials for revegetation and restoration (McKay et al. 2005). For example, positive findings of adaptation to local selective pressures (Joshi et al. 2001; Leimu and Fischer 2008; Hereford 2009) have been used as an argument in favor of primarily using local ecotypes in restoration (USDI and USDA 2002; Johnson et al. 2010a; Vander Mijnsbrugge et al. 2010b). Native plant material choices impact the viability and adaptive potential of restored populations (Williams 2001; Broadhurst et al. 2006; Aavik et al. 2012), as well as the feasibility of using locally collected seeds in large‐scale restoration (Merritt and Dixon 2011). Because of this, it is critical to understand the extent to which LAR can be broadly applied to land management.
Findings of LAR have substantially advanced our understanding of local adaptation in plants, yet it remains unclear to what extent the methods used in previous LAR allow us to assess the magnitude of local adaptation at a scale relevant to land management. Three issues could complicate the application of LAR to management questions. (i) Although LAR aims to understand adaptation at the metapopulation level, the number of populations and habitats sampled is typically small; (ii) the ability to apply findings to restoration at the landscape scale depends in large part on whether experimental methodologies capture selective pressures at relevant temporal and spatial scales; (iii) in addition, the experimental environment, response variables selected and maternal effects all affect the extent to which one can apply LAR to native plant material choices. Given these issues, we conducted a literature review to assess to what extent the methodology of LAR can be extrapolated to inform land managers about the choice of best native plant material for restoration. Specifically, we assessed six experimental methodologies:
Experimental environment—The most conclusive method for detecting local adaptation is through replicated reciprocal transplant experiments that compare fitness in multiple home and foreign sites (Kawecki and Ebert 2004; Blanquart et al. 2013). Multiple sites allow researchers to identify traits related to fitness that have been selected by the environment. LAR will be informative for land management if experiments use whole environments (Nuismer and Gandon 2008), and occur at multiple sites and in experimental conditions that are similar to those found during revegetation. Under this scenario, researchers would gain insight into the scale of population differentiation and the frequency of local adaptation. By contrast, experiments conducted at single sites, such as common garden studies, can only show phenotypic variation among populations, not whether fitness is higher for local versus nonlocal populations. Common garden or greenhouse studies could help identify which population will perform best at a specific revegetation site, but in the majority of cases, native plant materials will be used at multiple sites with unknown conditions. Additionally, common garden and reciprocal transplant studies can be used in conjunction with gaining deeper insight into the drivers of local adaptation (Nuismer and Gandon 2008), but the usage of unaltered field environments is especially important to accurately assess fitness when local adaptation is only observed under specific environmental conditions (cryptic adaptation), such as the presence of native plant community competitors (Knight and Miller 2004; Bischoff et al. 2006; Rice and Knapp 2008).
Measures of response—From a restoration perspective, population growth rate is the most relevant direct fitness measure because it indicates long‐term population viability (Menges 1990; Rice and Emery 2003). Unlike individual trait measurements, such as biomass or reproductive success, multiplicative population growth rate incorporates multiple parameters related to population persistence and growth. One common metric for assessing population growth rate is lambda (λ), the proportional change in population size from one generation to the next; simply put, λ must be ≥1 for a population to persist. Plant traits that respond to selection in the populations' home sites can be also used to detect evidence of local adaptation, but they are less likely to be directly related to fitness and may not show a signal for response to selection. Furthermore, ecological restoration benefits from research conducted across multiple life‐history stages and generations, as fitness responses can vary across these scales (Donovan and Ehleringer 1992; Kelly 1992; Rice and Knapp 2008). Given that the majority of revegetation projects rely on seeds to establish native plants (Koch 2007; Broadhurst et al. 2008), research that focuses the expression and magnitude of local adaptation during germination and establishment may provide especially important information for land management.
Maternal effects—Observed phenotypic differences among populations can result from differences among genotypes (local adaptation) or maternal effects (Roach and Wulff 1987). Adaptive maternal effects have been found to increase performance of the progeny of maternal plants exposed to drought (Sultan et al. 2009), herbivory (Agrawal 2001, 2002), herbicide (Bozorgipour and Snape 1997) and shading (Donohue and Schmitt 1999; Galloway and Etterson 2007; Bell and Galloway 2008) in these environments. In addition, the effects on phenotype of progeny can persist for multiple generations (Miao et al. 1991). For populations that remain in place in the landscape, maternal effects may make fitness in sympatry even stronger (Espeland and Rice 2012). In the case of land management, however, seeds are moved away from the maternal plant environment and expected to show the same traits and performance. Maternal effects will not mask local adaptation when it is present, but they may be confused with local adaption (when it is absent) or inflate the observed magnitude of fitness differences (when it is present). When maternal effects drive adaptive plant traits and when maternal environments (i.e. seed production farms) differ from target environments, determining whether traits are the result of maternal effects or local adaption will be critical for predicting seed and plant performance in revegetation.
Number of populations and habitats—Assessing the spatial scale of environmental and genetic differentiation requires sampling many individuals and populations (Manel et al. 2003), especially if there is significant variation among populations. Just as populations differ in the selective pressures they experience, they also differ in the magnitude and direction of response to those pressures (Thompson et al. 2002; Leger and Espeland 2010), and populations may show fitness differences unrelated to local adaptation due to habitat quality or genetic factors such as inbreeding (Blanquart et al. 2013). These issues combine to make it difficult to determine which selective factors are important drivers of adaptive trait differentiation and the scale over which they operate. Additionally, the type and number of habitats sampled from influences the scale at which local adaptation can be assessed. When planning a revegetation project, the practitioner calculates the likelihood of differential genotypic success in the environment; using multiple populations collected from many habitats in LAR enhances the ability of practitioners to make these difficult decisions by clearly defining the magnitude, scale and drivers of local adaptation. The popularity of genecological studies that measure hundreds of field‐collected populations in common gardens to generate geographic limits of appropriate seed transfer (e.g. Johnson et al. 2010b; St Clair et al. 2013) is evidence that this magnitude of population sampling may be necessary to assist practitioners in seed selection.
Selective agents—Understanding the factors that drive population differentiation is important in choosing native plant materials. Plant species can be adapted to both abiotic conditions (e.g. soil and climate; Macel et al. 2007; Goransson et al. 2009) and biotic factors (e.g. pollinators and soil pathogens; Svenning et al. 1991; Thrall et al. 2002; Streisfeld and Kohn 2007), and interactions between factors can alter the observance or strength of local adaptation (Hufford et al. 2008; Lau et al. 2008). Understanding the impact of multiple selective factors on population fitness will not only help managers identify which factors define ‘local’, but also provides information about the field conditions under which higher home‐site fitness is observed. As ecological restoration and land management are carried out in the realm of communities and ecosystems, research needs to take a multitude of selective factors and their interactions into account.
Environmental variability—Beyond biotic and abiotic factors that are largely consistent across years, factors that vary across time can also be important agents of selection. For example, selective agents that drive local adaptation may only act on some generations of the target species (Rice and Mack 1991; Geber and Griffen 2003; Thompson et al. 2007) and impacts on nonlocal sources may not be apparent for decades (Millar and Libby 1989). Spatial variation is often used in ecological experiments to predict what would occur over a longer time span (Haubensak and Parker 2004) because temporally rare events required for the expression of local adaptation—such as disease or drought—are more likely to be captured when multiple sites are used. Therefore, the number of environments and the type of variation encompassed within LAR (either by conducting an experiment over multiple experiment years or using many sites) is important for assessing the constancy of the expression of local adaptation and the comparative risk of using nonlocal genotypes.
To date, reviews of LAR have focused on identifying the overall frequency and drivers of local adaptation (Leimu and Fischer 2008; Hereford 2009, 2010) or on best practices for researching local adaptation (Kawecki and Ebert 2004; Kawecki et al. 2012; Blanquart et al. 2013). There is an additional need to assess the extent to which existing LAR can inform decisions regarding genetically appropriate plant materials for land management; these decisions require an understanding of how selection across the landscape shapes plant traits that are most important for restoration establishment and long‐term success. In addition to genetic diversity in quantitative trait loci, local adaptation is an important consideration for successful revegetation, and policy and practice are increasingly focusing on using it to select where to collect and move plant materials. We conducted a literature review in order to quantify to what extent LAR has integrated six key methodological considerations and can guide choices of native plant materials for management.
Materials and methods
We performed a literature search in ISI Web of Science using the search terms ‘local adapt*’ and ‘plant*’, for the period of 1965 to February 2013. A total of 1046 studies were identified. We reviewed titles, abstracts and keywords of each article to determine suitability for inclusion and excluded studies that did not focus on local adaptation in vascular plants (439 studies), had primary species of interest that were non‐native invasive species (113 studies), used only molecular analysis (93 studies), focused on crop plant(s) (42 studies) or were not experimental (e.g. theoretical, modeling and review papers; 124 studies). If a study was comprised of multiple experiments, we recorded data on each experiment individually. The final analysis comprised 234 articles describing 308 experiments. The experiments tested for local adaptation in 278 different plant species, mostly forbs (69%) and graminoids (20%) and, to a lesser degree, trees (9%) and shrubs (2%). Of the nontree species, 74% were perennial and 26% were annual.
For each experiment, we assessed six methodological variables that are relevant for ecological restoration: experimental environment, measures of response, maternal effects, among‐population variability, selective agents, and spatial and temporal variability. We recorded components of the experimental environment (type of experiment, site type, inclusion of the home plant community) as well as the response variables analyzed (the life stages studied, whether data were collected over the plant's entire lifespan, and whether multiple generations were studied). To classify the extent to which experiments controlled maternal effects, we recorded whether plant materials used in each study were the result of collections from a controlled environment, or if authors accounted for maternal effects using early‐stage measurements (initial seed weight or initial plant size) as covariates in statistical analysis; these methods are commonly accepted and utilized to control for maternal effects as seed weight and plant size can be indicative of maternal provisioning. We also recorded the number of different habitat types that populations were collected from as reported by authors (e.g. grassland and dune sites, inland and coastal sites) and the number of different populations from which plant material was collected (defined by authors). We identified the type and number of agents of natural selection that were tested within each experiment (biotic interactions and abiotic factors). To determine the spatial and temporal variability captured in experimental design, we recorded the duration of each experiment (rounded to the nearest year), the number of environments that were used in studies that were done in unmanipulated field conditions, or the number of experimental conditions tested whether investigators used treatments to create multiple experimental environments.
Results
Experimental environment
Thirty‐nine percent of experiments used reciprocal field transplants among the populations' home sites, whereas 33% used common garden designs (Table 1). Roughly half (N = 55) of the common garden experiments were conducted at a single site. Greenhouse and growth chamber experiments were the least frequently used (28%, N = 87). Approximately equal numbers of experiments were performed in natural sites (41%, N = 125) as in artificial settings (pots, greenhouses and growth chambers; Table 1). Sixty‐eight percent of experiments (N = 208; Table 1) removed local vegetation from the experimental environment.
Table 1.
Frequency (number and %) of use of six key experimental methodologies in local adaptation experiments (N = 308)
| Variable | Frequency | |
|---|---|---|
| No. | % | |
| Experimental environment | ||
| Experiment type | ||
| Reciprocal transplant | 120 | 39 |
| Common garden | 101 | 33 |
| Greenhouse | 87 | 28 |
| Site type | ||
| Natural site | 125 | 41 |
| Artificial conditions | 133 | 59 |
| Other vegetation included | ||
| Only target plant species present | 208 | 68 |
| Native vegetation intact or added | 78 | 25 |
| Measure of response | ||
| Fitness | ||
| Population growth rate (λ) | 14 | 5 |
| Reproductive success | 137 | 44 |
| Germination/emergence | 63 | 20 |
| Survival/mortality | 126 | 41 |
| Damage by herbivores/pathogens | 22 | 7 |
| Visitation from mutualists | 3 | 1 |
| Size (e.g. biomass, number of leaves, circumference) | 182 | 59 |
| Other | 46 | 15 |
| Life stages | ||
| Germination | 79 | 26 |
| Juvenile | 258 | 84 |
| Reproduction | 173 | 56 |
| 2 stages | 124 | 40 |
| All 3 stages | 41 | 13 |
| Multiple generations | ||
| Yes | 12 | 4 |
| No | 296 | 96 |
| Entire life cycle | ||
| Yes | 64 | 21 |
| No | 244 | 79 |
| Number of populations and habitats | ||
| Number of populations (mean) | 8 | – |
| Number of habitats plant material collected from (mean) | 3 | – |
| Maternal effects | ||
| Plant material from controlled environment | 89 | 29 |
| Weighed seeds | 37 | 12 |
| Kept maternal families separate | 50 | 16 |
| Initial plant size used as covariate | 51 | 17 |
| Selective agents | ||
| Biotic factors | ||
| Plant | 40 | 13 |
| Herbivore | 20 | 6 |
| Pathogen | 3 | 1 |
| Mutualist | 7 | 2 |
| Soil biota | 13 | 4 |
| Multiple biotic factors | 5 | 2 |
| Biotic and abiotic factors | 42 | 14 |
| Abiotic factors | ||
| Climate | 144 | 47 |
| Soil | 65 | 21 |
| Light | 10 | 3 |
| Disturbance | 31 | 10 |
| Distance | 3 | 1 |
| Other | 40 | 13 |
| Multiple abiotic factors | 27 | 9 |
| Environmental variability | ||
| Length of experiment (years; mean) | 2 | – |
| Number of sites or created environments (mean) | 4 | – |
Response variables
Although 82% of experiments calculated a measure of fitness, only 5% (N = 14) included λ as a response variable (Table 1). Biomass was the most frequently used measure of fitness (59%), followed by reproductive success (44%). The most common life‐history stage assessed was nonreproductive, followed by reproductive adult (Table 1); germination was the least commonly tracked (26%; Table 1). Forty‐one percent of experiments tracked two life stages, and 13% tracked plants across all three life stages (Fig. 1). The majority of studies did not follow plants until death (77%, N = 244; Table 1) or track multiple generations (96%, N = 296; Table 1).
Figure 1.
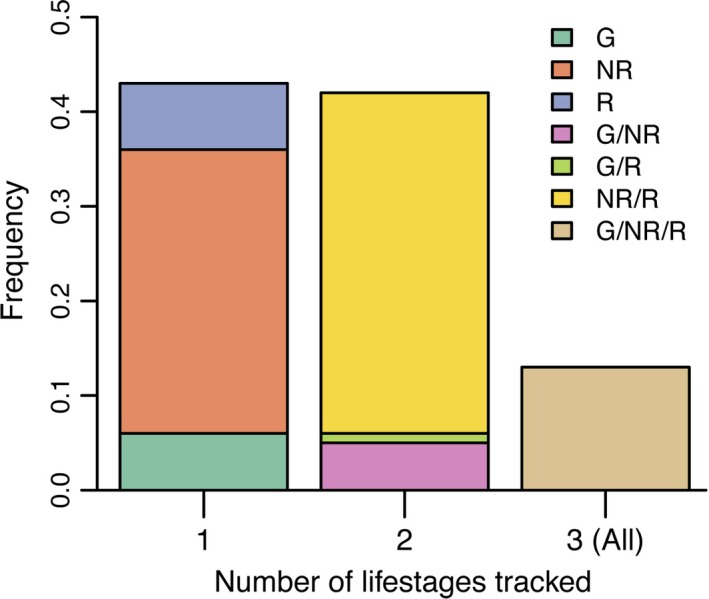
Frequency of local adaptation experiments (proportion; N = 308) that tracked plants during germination (G), nonreproductive juvenile or adult (NR), and reproductive (R) life stages, or combinations thereof.
Maternal effects
Approximately three quarters of experiments controlled for maternal effects in some way. However, most of these (45%, N = 138) used initial plant size or seed as a statistical covariate, or kept maternal families separate in statistical analysis (Table 1). Only a third (29%) included plant material that had been grown in a controlled maternal environment.
Among‐population variability
We found wide variation in the number of collection populations and habitats (Fig. 2). On average, experiments used plant materials collected from eight populations and three different habitat types.
Figure 2.
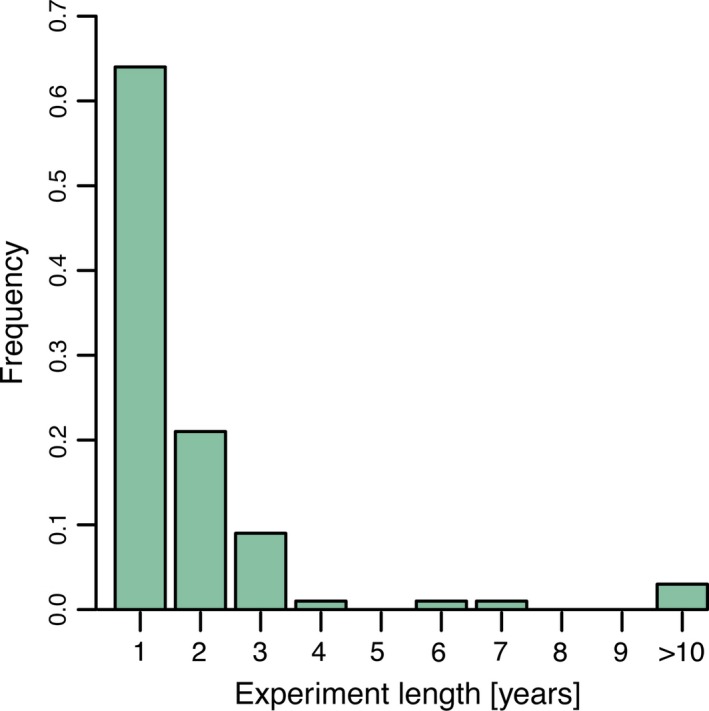
Frequency of local adaptation experiments (proportion, N = 308) by level of replication (none to >10) for populations (black bars) and habitats (white bars). Population was defined by authors as a single source of plant materials. Habitat refers to areas from which populations were collected.
Selective agents
The majority of experiments tested adaptation to abiotic factors (89%, N = 271). Biotic factors were rarely considered (25%, N = 76), and only 2% (N = 7) assessed adaptation in the presence of multiple biotic factors (Table 1). The majority of studies that tested abiotic factors focused on climate (Table 1). Additional factors were overall ecological and geographic differences between populations, salt‐spray tolerance and inundation gradients (Table 1). Ten percent (N = 27) of studies tested adaptation to multiple abiotic factors or abiotic and biotic factors in combination (14%, N = 42).
Spatial and temporal variability
On average, experiments ran for 2 years, with the median being <1 year (Fig. 3). The longest running experiment lasted 45 years (Gomory et al. 2012). There was a wide range of variability in the number of environments experiments occurred in (sites or environmental conditions if a greenhouse or common garden study; mean = 4, median = 3; Table 1).
Figure 3.
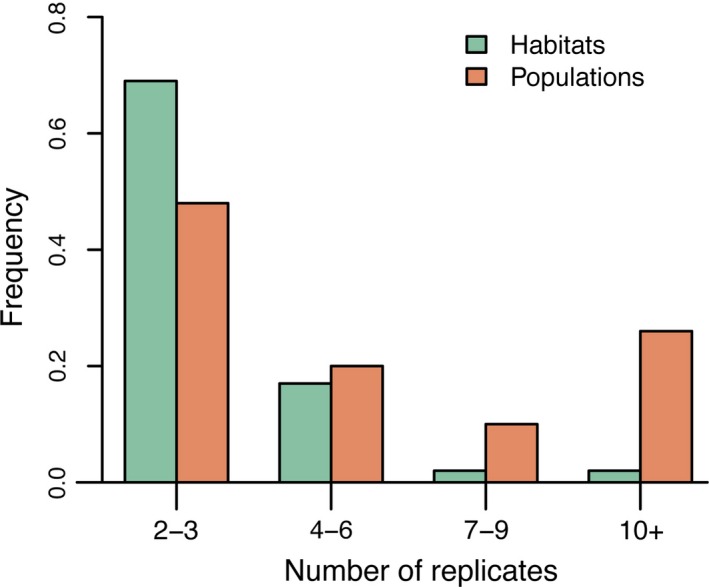
Frequency of local adaptation experiments (proportion; N = 308) by experimental duration in years.
Discussion
Practitioners have increasingly used results from LAR to guide management decisions (e.g. Vander Mijnsbrugge et al. 2010a). However, our results suggest that findings from LAR are not easily transferable to land management due to experimental constraints. In particular, LAR primarily used experimental environments that did not mimic natural conditions, chose response variables that did not reflect lifetime fitness, excluded biotic and multiple selective factors, and used limited replication and experimental duration. While these methodological choices do not reflect the quality or findings of individual experiments designed to test specific factors of interest, practitioners should interpret results from LAR with caution.
As with previous reviews (Leimu and Fischer 2008; Hereford 2009), we found that only a portion of LAR directly addresses local adaptation through the use of reciprocal transplant studies (39%). The frequencies of finding local adaptation do not appear to differ between common garden and reciprocal transplant studies (Leimu and Fischer 2008), but observed differences among populations can differ from common garden studies in strength (Table 2). Perhaps more importantly for applying LAR, less than half of studies occurred in natural environments (41%), or retained the native plant community (25%). Although removing confounding factors such as natural site variation and the home plant community can make it easier to study factors of interest, it impacts both probably of detection and whether findings are relevant in situ (McCarragher et al. 2011; Ehlers et al. 2012; Pankova et al. 2014; Table 2). Furthermore, the choice of traits or inclusion of λ in LAR is relevant for whether findings of higher fitness translate to increased population persistence, and the two may give contradictory results that alter whether local adaptation is observed (Table 2). We found that only 40% of LAR used direct fitness measures (either survival or reproductive success) and very few (5%) used λ. Incorporating multiple life stages increases the applicability of LAR to management as the use of local native plant materials is often predicated on the assumption that local adaptation will increase population fitness at critical life stages, yet local populations may not show consistent trends of higher fitness across their entire life cycle (Table 2). Germination and emergence are often the limiting factors in revegetation success (Khurana and Singh 2001; Pywell et al. 2003) and critical to population regulation (Horvitz and Schemske 1995; Freville and Silvertown 2005); however, less than a quarter of experiments incorporated these life stages as a measure of fitness.
Table 2.
Examples of local adaptation experiments that incorporated variables that are informative to ecological restoration, and a brief summary of the impact of the variable on the findings of local adaptation or population differentiation. Papers did not incorporate all six variables equally, and summary findings could be influenced by the remaining five variables
| Variable | Authors | Summary |
|---|---|---|
| Reciprocal versus common garden | Raabova et al. (2011) | Results from reciprocal transplant and common garden experiments differed in the observed level of population differentiation. While both types of experiments showed greater height of local versus foreign plants, there were smaller differences in height in the field compared to the common garden. This indicates that the magnitude of difference was smaller in the reciprocal transplant compared to common garden experiment |
| Inclusion of native vegetation | Bischoff et al. (2006) | Inclusion or exclusion of the local plant community altered the detection and magnitude of local adaptation in two species. Fitness was higher for Plantago lanceolata when the native plant community was present, while Holcus lanatus showed lower home‐site fitness with the local plant community present |
| Population growth rate (λ) | Becker et al. (2006) | Findings about population fitness were different when fitness in traits and lifetime fitness (λ) were assessed. Four of six life‐history traits studied showed nonsignificant differences between home versus away populations; however, λ showed a significant home‐site advantage |
| Multiple life stages | Raabova, Muenzbergova and Fischer (2007) | Findings of local adaptation depended on life stage assessed. Evidence of local adaptation was seen in the number of germinates (up to 68% higher in local versus foreign populations), but no consistent evidence of local adaptation was found in adults |
| Multiple populations/habitats | Hereford and Winn (2008) | Evidence of home‐site advantage was rare and depended on the degree of habitat similarity. Local adaptation was not found when populations were from the same habitat type, but was significantly likely to be found when populations were from different habitats |
| Plant materials from controlled environment | Bischoff and Muller‐Scharer (2010) | Maternal effects impacted level of population differentiation detected and observed traits. Populations showed less differentiation when using plants from controlled crosses than parent plants. The ranking of populations in the F1 generation also changed for some traits. Maternal effects were independent of seed mass |
| Multiple factors | Lau (2006) | Findings of adaptation varied when multiple biotic factors versus a single factor were studied. When grown only with the invasive Medicago polymorpha, Lotus wrangelianus plants from invaded sites showed adaptation to invasion. There was no evidence of adaptation to the invader when the insect herbivore Hypera brunneipennis was included |
| Experimental length | Bennington et al. (2012) | Experimental length was important for the observation and magnitude of local adaptation. For Dryas octopetala, the strength of local adaptation increased over a decade. For Eriophorum vaginatum, there was no evidence of local adaptation until 17 years after transplant |
Reciprocal transplants and direct fitness measures are just two of the important experimental considerations for applying LAR to restoration; given the expense of using local seeds, managers need to be confident that local sources will result in long‐term increased fitness in restored populations. Replication over space and time and the inclusion of relevant selective agents are equally important, but rarely adequately addressed. Thus, it is unknown whether findings of local adaptation are due to fitness differences in response to selective agents or trait differentiation unrelated to fitness, and it could be additionally difficult to determine whether local seeds will be consistent in showing higher fitness under altered site conditions (Table 2). The limited number of habitats plant materials was collected from increases the risk that LAR has selectively used populations from a few highly contrasting environments, thereby increasing the chance of finding fitness differences regardless of experimental methodology used (Hereford and Winn 2008; Hereford 2009; Table 2), directly limiting the application of LAR to decisions regarding the scale and importance of local adaptation in choosing plant materials. In addition, Siepielski et al. (2009) found that the strength, direction and sources of selection frequently change among years (but see Morrissey and Hadfield 2012)—the short duration and limited testing conditions of most LAR indicate that even normal variation at experimental sites is unlikely to be captured. The magnitude fitness differences due to local adaptation can change over decades (Table 2), leaving the question of whether short duration research accurately represents the population dynamics that will occur postrevegetation.
One essential consideration that was frequently addressed in LAR was maternal effects. Although Hereford (2009) anecdotally noted that most LAR experiments did not account for them, we found that 74% of experiments controlled for maternal effects in some way, although only 29% used plant materials from common environments. Maternal effects can increase the observed differences among populations (Table 2) and could alter the interpretation of higher fitness. The frequent use of measures to control for maternal effects suggests that most LAR does not confound transgenerational plasticity and genetic differentiation. In this aspect, LAR can be appropriately applied to problems of moving genotypes from one environment to another.
Future direction
The difficulty of conducting LAR that can be applied to management may in part stem from logistical obstacles in research and dependence on short‐term funding. For instance, the inclusion of λ as a response variable is complicated by that fact that: (i) extended periods of data collection are required to accurately estimate it for long‐lived species (Che‐Castaldo and Inouye 2011); and (ii) that estimates of λ in plants require accounting for factors such as seed banks (Adams et al. 2005), dormancy (Miller et al. 2012) and nonseed reproduction (Nault and Gagnon 1993). It can also be difficult to study multiple selective factors in concert or to determine which selective agents are important in natural field settings.
Even though ideal experimental considerations are likely unattainable, investigators interested in research for restoration application could address a greater set of considerations in their designs (Fig. 4). First, they could increase the number of populations and the sites and life‐history stages assessed, and increase study duration. Second, if utilizing λ is not feasible, researchers could test for fitness differences in response to selective agents at specific life‐history stages concurrently, rather than sequentially. Third, performing LAR over environmental gradients or clines (Etterson 2004; Fant et al. 2008) has the advantage of determining the importance of landscape variability over multiple scales on the expression of local adaptation. Researchers could increase their participation in inter‐regional or intercontinental collaboration to allow the inclusion of more populations and habitats in local adaptation experiments. Alternatively, researchers and managers could increase their collaboration by tracking the success of locally collected seeds at restoration sites. Finally, combining reciprocal transplants in natural conditions with controlled common garden experiments could provide greater information about the drivers and magnitude of local adaptation (Raabova et al. 2011). These suggestions are valid for all LAR and would help researchers adhere to best practice. Results from experiments that included these six factors illustrate their importance in assessing local adaptation, and managers should consider how directly LAR could inform policy.
Figure 4.
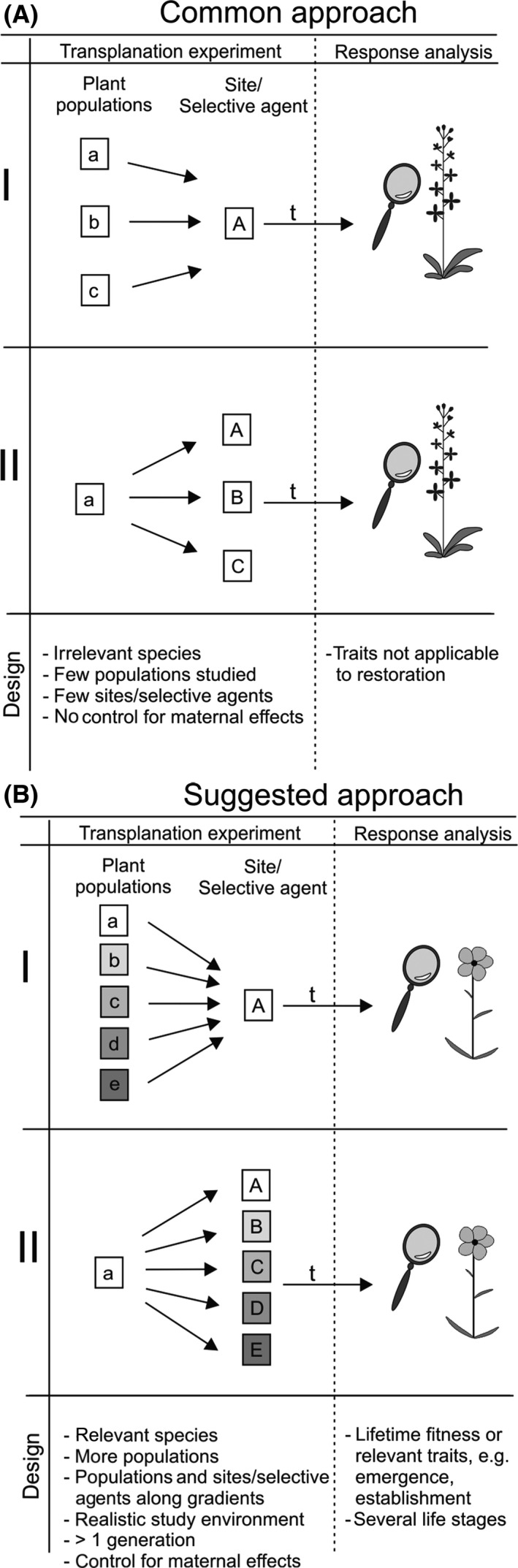
Schematic graph of the (A) common approach to local adaptation experiments and (B) a suggested approach that could make experiments more relevant to land management. Small letters (a–e) indicate plant populations; capital letters (A–E) indicate sites or selective agents; and t indicates time after the beginning of the experiment. In (B), gray‐shaded colors, underlying boxes (a–e, A–E) represent an environmental or geographic gradient. In panel a, material from multiple plant populations is crossed either at one site (I) or with one selective agent (II). In panel b, material from multiple plant populations is crossed with multiple sites or selective agents. Dots indicate that reciprocal transplant is replicated at the remaining sites.
Data accessibility
Data for this study were collected from peer‐reviewed published literature.
Acknowledgements
This research was supported by agreement #09‐CS‐11015600‐031 from the USDA Forest Service NFN program and the National Science Foundation EPSCoR program (# EPS‐1101342) at the University of Montana. VW was supported by a postdoctoral fellowship by the Alexander von Humboldt Foundation. Thanks to Drs. Ray Callaway, Lila Fishman, Elizabeth Crone, Dean Pearson and Solomon Dobrowski for feedback.
Literature cited
- Aavik, T. , Edwards P. J., Holderegger R., Graf R., and Billeter R. 2012. Genetic consequences of using seed mixtures in restoration: a case study of a wetland plant Lychnis flos‐cuculi . Biological Conservation 145:195–204. [Google Scholar]
- Adams, V. M. , Marsh D. M., and Knox J. S. 2005. Importance of the seed bank for population viability and population monitoring in a threatened wetland herb. Biological Conservation 124:425–436. [Google Scholar]
- Agrawal, A. A. 2001. Transgenerational consequences of plant responses to herbivory: an adaptive maternal effect? The American Naturalist 157:555–569. [DOI] [PubMed] [Google Scholar]
- Agrawal, A. A. 2002. Herbivory and maternal effects: mechanisms and consequences of transgenerational induced plant resistance. Ecology 83:3408–3415. [Google Scholar]
- Barrett, S. C. H. , Colautti R. I., and Eckert C. G. 2008. Plant reproductive systems and evolution during biological invasion. Molecular Ecology 17:373–383. [DOI] [PubMed] [Google Scholar]
- Becker, U. , Colling G., Dostal P., Jakobsson A., and Matthies D. 2006. Local adaptation in the monocarpic perennial Carlina vulgaris at different spatial scales across Europe. Oecologia 150:506–518. [DOI] [PubMed] [Google Scholar]
- Bell, D. L. , and Galloway L. F. 2008. Population differentiation for plasticity to light in an annual herb: adaptation and cost. American Journal of Botany 95:59–65. [DOI] [PubMed] [Google Scholar]
- Bennington, C. C. , Fetcher N., Vavrek M. C., Shaver G. R., Cummings K. J., and McGraw J. B. 2012. Home site advantage in two long‐lived arctic plant species: results from two 30‐year reciprocal transplant studies. Journal of Ecology 100:841–851. [Google Scholar]
- Bischoff, A. , and Muller‐Scharer H. 2010. Testing population differentiation in plant species ‐ how important are environmental maternal effects. Oikos 119:445–454. [Google Scholar]
- Bischoff, A. , Cremieux L., Smilauerova M., Lawson C. S., Mortimer S. R., Dolezal J., Lanta V. et al. 2006. Detecting local adaptation in widespread grassland species – the importance of scale and local plant community. Journal of Ecology 94:1130–1142. [Google Scholar]
- Blanquart, F. , Kaltz O., Nuismer S. L., and Gandon S. 2013. A practical guide to measuring local adaptation. Ecology Letters 16:1195–1205. [DOI] [PubMed] [Google Scholar]
- Bozorgipour, R. , and Snape J. W. 1997. An assessment of somaclonal variation as a breeding tool for generating herbicide tolerant genotypes in wheat (Triticum aestivum L.). Euphytica 94:335–340. [Google Scholar]
- Broadhurst, L. M. , North T., and Young A. G. 2006. Should we be more critical of remnant seed sources being used for revegetation? Ecological Management & Restoration 7:211–217. [Google Scholar]
- Broadhurst, L. M. , Lowe A., Coates D. J., Cunningham S. A., McDonald M., Vesk P. A., and Yates C. 2008. Seed supply for broadscale restoration: maximizing evolutionary potential. Evolutionary Applications 1:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che‐Castaldo, J. P. , and Inouye D. W. 2011. The effects of dataset length and mast seeding on the demography of Frasera speciosa, a long‐lived monocarpic plant. Ecosphere 2:18. [Google Scholar]
- Donohue, K. , and Schmitt J. 1999. The genetic architecture of plasticity to density in Impatiens capensis . Evolution 53:1377–1386. [DOI] [PubMed] [Google Scholar]
- Donovan, L. A. , and Ehleringer J. R. 1992. Contrasting water‐use patterns among size and life‐history classes of a semiarid shrub. Functional Ecology 6:482–488. [Google Scholar]
- Ehlers, B. K. , Grondahl E., Ronfort J., and Bataillon T. 2012. “Menage a trois”: the presence/absence of thyme shapes the mutualistic interaction between the host plant Medicago truncatula (Fabaceae) and its symbiotic bacterium Sinorhizobium meliloti . Ecology and Evolution 2:1676–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland, E. K. , and Rice K. J. 2012. Within‐ and trans‐generational plasticity affects the opportunity for selection in barbed goatgrass (Aegilops triuncials). American Journal of Botany 99:2058–2062. [DOI] [PubMed] [Google Scholar]
- Etterson, J. R. 2004. Evolutionary potential of Chamaecrista fasciculata in relation to climate change. 1. Clinal patterns of selection along an environmental gradient in the great plains. Evolution 58:1446–1458. [DOI] [PubMed] [Google Scholar]
- Fant, J. B. , Holmstrom R. M., Sirkin E., Etterson J. R., and Masi S. 2008. Genetic structure of threatened native populations and propagules used for restoration in a clonal species, American beachgrass (Ammophila breviligulata Fern.). Restoration Ecology 16:594–603. [Google Scholar]
- Freville, H. , and Silvertown J. 2005. Analysis of interspecific competition in perennial plants using life table response experiments. Plant Ecology 176:69–78. [Google Scholar]
- Galloway, L. F. , and Etterson J. R. 2007. Transgenerational plasticity is adaptive in the wild. Science 318:1134–1136. [DOI] [PubMed] [Google Scholar]
- Geber, M. A. , and Griffen L. R. 2003. Inheritance and natural selection on functional traits. International Journal of Plant Sciences 164:S21–S42. [Google Scholar]
- Gomory, D. , Longauer R., Hlasny T., Pacalaj M., Strmen S., and Krajmerova D. 2012. Adaptation to common optimum in different populations of Norway spruce (Picea abies Karst.). European Journal of Forest Research 131:401–411. [Google Scholar]
- Goransson, P. , Andersson S., and Falkengren‐Grerup U. 2009. Genetic adaptation to soil acidification: experimental evidence from four grass species. Evolutionary Ecology 23:963–978. [Google Scholar]
- Haubensak, K. A. , and Parker I. M. 2004. Soil changes accompanying invasion of the exotic shrub Cytisus scoparius in glacial outwash prairies of western Washington USA. Plant Ecology 175:71–79. [Google Scholar]
- Hereford, J. 2009. A quantitative survey of local adaptation and fitness trade‐offs. The American Naturalist 173:579–588. [DOI] [PubMed] [Google Scholar]
- Hereford, J. 2010. Does selfing or outcrossing promote local adaptation? American Journal of Botany 97:298–302. [DOI] [PubMed] [Google Scholar]
- Hereford, J. , and Winn A. A. 2008. Limits to local adaptation in six populations of the annual plant Diodia teres . New Phytologist 178:888–896. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , and Sgro C. M. 2011. Climate change and evolutionary adaptation. Nature 470:479–485. [DOI] [PubMed] [Google Scholar]
- Horvitz, C. C. , and Schemske D. W. 1995. Spatiotemporal variation in demographic transitions of a tropical understory herb – projection matrix analysis. Ecological Monographs 65:155–192. [Google Scholar]
- Hufford, K. M. , and Mazer S. J. 2003. Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends in Ecology & Evolution 18:147–155. [Google Scholar]
- Hufford, K. M. , Mazer S. J., and Camara M. D. 2008. Local adaptation and effects of grazing among seedlings of two native california bunchgrass species: implications for restoration. Restoration Ecology 16:59–69. [Google Scholar]
- Johnson, N. C. , Wilson G. W. T., Bowker M. A., Wilson J. A., and Miller R. M. 2010a. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proceedings of the National Academy of Sciences of the USA 107:2093–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. C. , Erickson V. J., Mandel N. L., St Clair J. B., and Vance‐Borland K. W. 2010b. Mapping genetic variation and seed zones for Bromus carinatus in the Blue Mountains of eastern Oregon, USA. Botany‐Botanique 88:725–736. [Google Scholar]
- Jordan, D. S. 1905. The origin of species through isolation. Science 22:545–562. [DOI] [PubMed] [Google Scholar]
- Joshi, J. , Schmid B., Caldeira M. C., Dimitrakopoulos P. G., Good J., Harris R., Hector A. et al. 2001. Local adaptation enhances performance of common plant species. Ecology Letters 4:536–544. [Google Scholar]
- Kawecki, T. J. , and Ebert D. 2004. Conceptual issues in local adaptation. Ecology Letters 7:1225–1241. [Google Scholar]
- Kawecki, T. J. , Lenski R. E., Ebert D., Hollis B., Olivieri I., and Whitlock M. C. 2012. Experimental evolution. Trends in Ecology & Evolution 27:547–560. [DOI] [PubMed] [Google Scholar]
- Kelly, C. A. 1992. Spatial and temporal variation in selection on correlated life‐history traits and plant size in Chamaecrista fasciculata . Evolution 46:1658–1673. [DOI] [PubMed] [Google Scholar]
- Khurana, E. , and Singh J. S. 2001. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: a review. Environmental Conservation 28:39–52. [Google Scholar]
- Knight, T. M. , and Miller T. E. 2004. Local adaptation within a population of Hydrocotyle bonariensis . Evolutionary Ecology Research 6:103–114. [Google Scholar]
- Koch, J. M. 2007. Restoring a Jarrah forest understorey vegetation after bauxite mining in Western Australia. Restoration Ecology 15:S26–S39. [Google Scholar]
- Lau, J. A. 2006. Evolutionary responses of native plants to novel community members. Evolution 60:56–63. [PubMed] [Google Scholar]
- Lau, J. A. , McCall A. C., Davies K. F., McKay J. K., and Wright J. W. 2008. Herbivores and edaphic factors constrain the realized niche of a native plant. Ecology 89:754–762. [DOI] [PubMed] [Google Scholar]
- Leger, E. A. , and Espeland E. K. 2010. Coevolution between native and invasive plant competitors: implications for invasive species management. Evolutionary Applications 3:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimu, R. , and Fischer M. 2008. A meta‐analysis of local adaptation in plants. PLoS ONE 3:e4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macel, M. , Lawson C. S., Mortimer S. R., Smilauerova M., Bischoff A., Cremieux L., Dolezal J. et al. 2007. Climate vs. soil factors in local adaptation of two common plant species. Ecology 88:424–433. [DOI] [PubMed] [Google Scholar]
- Manel, S. , Schwartz M. K., Luikart G., and Taberlet P. 2003. Landscape genetics: combining landscape ecology and population genetics. Trends in Ecology & Evolution 18:189–197. [Google Scholar]
- McCarragher, S. R. , Goldblum D., and Rigg L. S. 2011. Geographic variation of germination, growth, and mortality in sugar maple (Acer saccharum): common garden and reciprocal dispersal experiments. Physical Geography 32:1–21. [Google Scholar]
- McKay, J. K. , Christian C. E., Harrison S., and Rice K. J. 2005. “How local is local?” – a review of practical and conceptual issues in the genetics of restoration. Restoration Ecology 13:432–440. [Google Scholar]
- Menges, E. S. 1990. Population viability analysis for an endangered plant. Conservation Biology 4:52–62. [Google Scholar]
- Merritt, D. J. , and Dixon K. W. 2011. Restoration seed banks – a matter of scale. Science 332:424–425. [DOI] [PubMed] [Google Scholar]
- Miao, S. L. , Bazzaz F. A., and Primack R. B. 1991. Persistence of maternal nutrient effects in Plantago major – the 3rd generation. Ecology 72:1634–1642. [Google Scholar]
- Millar, C. , and Libby W. 1989. Restoration: Disneyland or native ecosystem? A question of genetics. Restoration and Management Notes 7:18–23. [Google Scholar]
- Miller, M. T. , Antos J. A., and Allen G. A. 2012. Demography of a dormancy‐prone geophyte: influence of spatial scale on interpretation of dynamics. Plant Ecology 213:569–579. [Google Scholar]
- Morrissey, M. B. , and Hadfield J. D. 2012. Directional selection in temporally replicated studies is remarkably consistent. Evolution 66:435–442. [DOI] [PubMed] [Google Scholar]
- Nault, A. , and Gagnon D. 1993. Ramet demography of Allium tricoccum, a spring ephemeral, perennial forest herb. Journal of Ecology 81:101–119. [Google Scholar]
- Nuismer, S. L. , and Gandon S. 2008. Moving beyond common‐garden and transplant designs: insight into the causes of local adaptation in species interactions. The American Naturalist 171:658–668. [DOI] [PubMed] [Google Scholar]
- Pankova, H. , Raabova J., and Munzbergova Z. 2014. Mycorrhizal symbiosis and local adaptation in Aster amellus: a field transplant experiment. PLoS ONE 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pywell, R. F. , Bullock J. M., Roy D. B., Warman L. I. Z., Walker K. J., and Rothery P. 2003. Plant traits as predictors of performance in ecological restoration. Journal of Applied Ecology 40:65–77. [Google Scholar]
- Raabova, J. , Muenzbergova Z., and Fischer M. 2007. Ecological rather than geographic or genetic distance affects local adaptation of the rare perennial herb, Aster amellus . Biological Conservation 139:348–357. [Google Scholar]
- Raabova, J. , Munzbergova Z., and Fischer M. 2011. The role of spatial scale and soil for local adaptation in Inula hirta . Basic and Applied Ecology 12:152–160. [Google Scholar]
- Rice, K. J. , and Emery N. C. 2003. Managing microevolution: restoration in the face of global change. Frontiers in Ecology and the Environment 1:469–478. [Google Scholar]
- Rice, K. J. , and Knapp E. E. 2008. Effects of competition and life history stage on the expression of local adaptation in two native bunchgrasses. Restoration Ecology 16:12–23. [Google Scholar]
- Rice, K. J. , and Mack R. N. 1991. Ecological genetics of Bromus tectorum. 3. The demography of reciprocally sown populations. Oecologia 88:91–101. [DOI] [PubMed] [Google Scholar]
- Roach, D. A. , and Wulff R. D. 1987. Maternal effects in plants. Annual Review of Ecology and Systematics 18:209–235. [Google Scholar]
- Siepielski, A. M. , DiBattista J. D., and Carlson S. M. 2009. It's about time: the temporal dynamics of phenotypic selection in the wild. Ecology Letters 12:1261–1276. [DOI] [PubMed] [Google Scholar]
- St Clair, J. B. , Kilkenny F. F., Johnson R. C., Shaw N. L., and Weaver G. 2013. Genetic variation in adaptive traits and seed transfer zones for Pseudoroegneria spicata (bluebunch wheatgrass) in the northwestern United States. Evolutionary Applications 6:933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisfeld, M. A. , and Kohn J. R. 2007. Environment and pollinator‐mediated selection on parapatric floral races of Mimulus aurantiacus . Journal of Evolutionary Biology 20:122–132. [DOI] [PubMed] [Google Scholar]
- Sultan, S. E. , Barton K., and Wilczek A. M. 2009. Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology 90:1831–1839. [DOI] [PubMed] [Google Scholar]
- Svenning, M. M. , Junttila O., and Solheim B. 1991. Symbiotic growth of indigenous white clover (Trifolium repens) with local rhizobium Leguminosarum biovar trifolii . Physiologia Plantarum 83:381–389. [Google Scholar]
- Thompson, J. N. , Nuismer S. L., and Gomulkiewicz R. 2002. Coevolution and maladaptation. Integrative and Comparative Biology 42:381–387. [DOI] [PubMed] [Google Scholar]
- Thompson, J. D. , Gauthier P., Amiot J., Ehlers B. K., Collin C., Fossat J., Barrios V. et al. 2007. Ongoing adaptation to mediterranean climate extremes in a chemically polymorphic plant. Ecological Monographs 77:421–439. [Google Scholar]
- Thrall, P. H. , Burdon J. J., and Bever J. D. 2002. Local adaptation in the Linum marginale‐Melampsora lini host–pathogen interaction. Evolution 56:1340–1351. [DOI] [PubMed] [Google Scholar]
- USDI and USDA 2002. Report to the Congress: Interagency Program to Supply and Manage Native Plant Materials for Restoration and Rehabilitation on Federal Lands. USDI and USDA, Washington, DC, USA. [Google Scholar]
- Vander Mijnsbrugge, K. , Bischoff A., and Smith B. 2010a. A question of origin: where and how to collect seed for ecological restoration. Basic and Applied Ecology 11:300–311. [Google Scholar]
- Vander Mijnsbrugge, K. , De Cock K., Cox K., and Breyne P. 2010b. Conservation measures for Rosa arvensis Huds. in Flanders (Belgium) based on congruent genetic and phenotypic population differentiation. Conservation Genetics 11:2243–2253. [Google Scholar]
- Vitt, P. , Havens K., Kramer A. T., Sollenberger D., and Yates E. 2010. Assisted migration of plants: changes in latitudes, changes in attitudes. Biological Conservation 143:18–27. [Google Scholar]
- Williams, S. L. 2001. Reduced genetic diversity in eelgrass transplantations affects both population growth and individual fitness. Ecological Applications 11:1472–1488. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study were collected from peer‐reviewed published literature.


