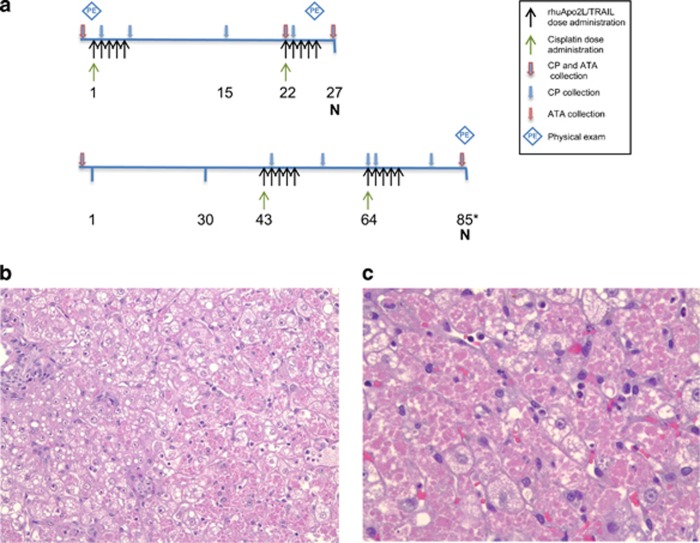
Figure 2.
Evidence of hepatotoxicity in two renal injury model studies using concomitant administration of cisplatin and rhuApo2L/TRAIL. (a) Study design schemas. Black arrows indicate rhuApo2L/TRAIL administration (top line: 0, 30 or 100 mg/kg rhuApo2/TRAIL (n=2/sex/group) on days 1–5 and 22–26; bottom line: 100 mg/kg rhuApo2/TRAIL on days 43–47 and 64–68 (n=6/sex)); green arrows indicate cisplatin administration (4 mg/kg; top line: days 1 and 22, bottom line: days 43 and 64); blue arrows outlined in red indicate timing of blood sampling for clinical pathology (CP) and ATA/crosslinking activity analyses; blue arrows indicate timing of blood sampling for CP analysis; red arrows indicate timing of blood sampling for ATA/crosslinking activity analyses. Blue diamonds indicate timing of physical examinations. N, necropsy (*day 85 or 86). (b) Representative hematoxylin and eosin (H&E)-stained liver section at × 20 magnification, and (c) representative H&E-stained section at × 40 magnification, depicting rhuApo2L/TRAIL-associated hepatic pathology in an animal in the concomitant cisplatin (4 mg/kg)+rhuApo2L/TRAIL (100 mg/kg) group. Disrupted hepatic architecture was observed and many vacuolated and necrotic hepatocytes were present