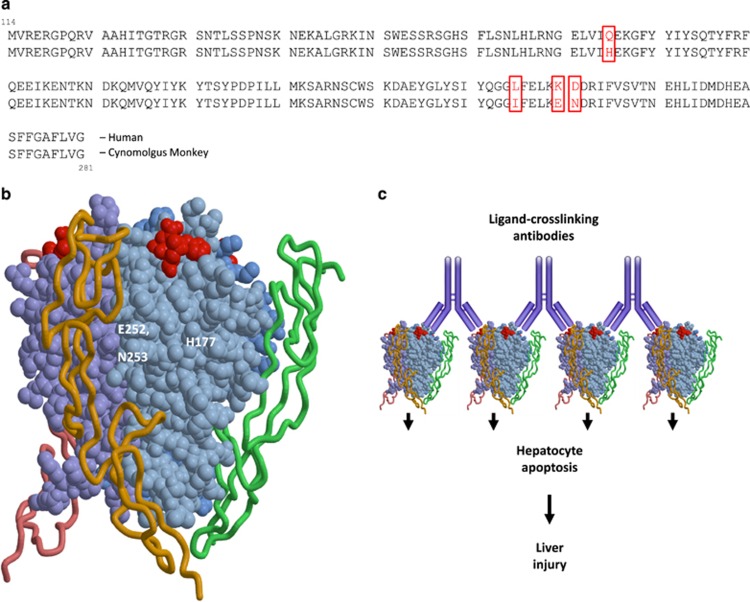
Figure 5.
(a) Sequence comparison of human and cynomolgus monkey Apo2L/TRAIL, residues 114–281 of the extracellular domain, showing the positions of the distinct amino acids between the two species. (b) Three-dimensional rendering of human Apo2L/TRAIL interacting with a membrane-bound death receptor. Shown in red are three of the four amino acids (H177, E252 and N253) that differ between the human and cynomolgus monkey proteins and are distal to the receptor binding region of the Apo2L/TRAIL protein. (c) A rendering of the mechanism of toxicity, wherein anti-Apo2L/TRAIL antibodies bind to Apo2L/TRAIL, bringing them into close proximity with one another and thereby clustering death receptors at the surface of the hepatocyte. Such receptor clustering can lead to excessive intracellular apoptosis signaling and consequent hepatotoxicity