Figure 1.
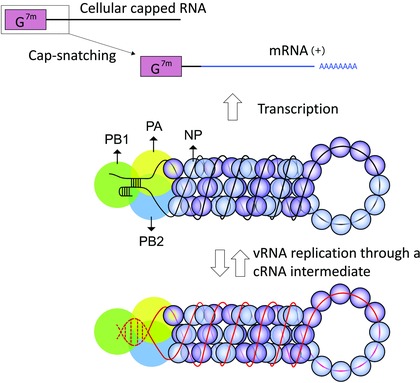
Role of the influenza virus polymerase complex in vRNA transcription and replication.
The influenza polymerase is a complex containing the PA, PB1, and PB2 proteins. One polymerase heterotrimer is attached to each vRNP segment inside the virion. These vRNPs have a double‐helical hairpin structure consisting of two antiparallel vRNA strands that are coated by NP molecules.17, 18
Transcription of vRNA to mRNA starts with the “cap‐snatching” reaction, in which cellular capped RNAs are bound by PB2 and cleaved, by PA, at 10–15 nucleotides from the cap, to yield primers for viral mRNA synthesis.19, 20 Termination and polyadenylation occur at a stretch of five to seven U residues near the 5ʹ end of the vRNA.21, 22 Replication of vRNA proceeds through full‐length complementary cRNAs, which are assembled as cRNP complexes. In both transcription and replication, RNA elongation is carried out by the PB1 subunit. The vRNA promoter is depicted as observed in the promoter‐bound polymerase crystal structures, in which its 5ʹ end forms a “hook” structure.23, 24 The cRNA promoter is drawn in dashed lines, since its conformation is yet to be determined. (Adapted from Portela et al.25)
