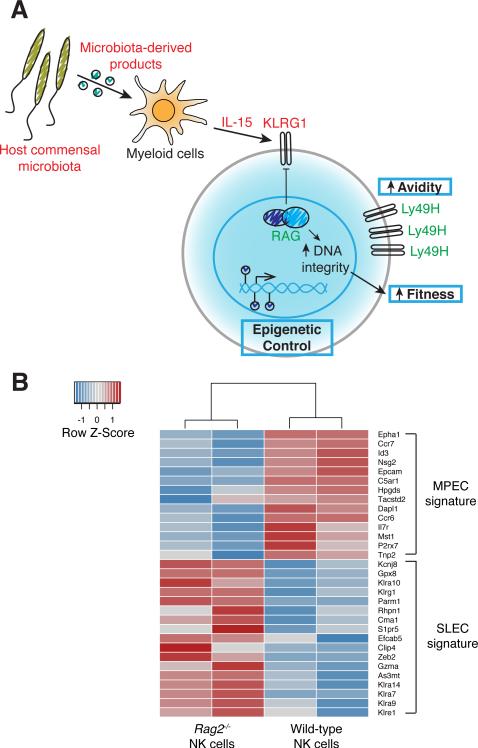
FIGURE 2.
Functional heterogeneity within the effector NK cell pool. A, Multiple mechanisms regulate memory precursor identity in NK cells. KLRG1 expression inversely correlates with NK cell memory potential. RAG expression during ontogeny not only negatively regulates KLRG1 expression but also promotes enhanced NK cell fitness by supporting optimal expression of DNA damage repair enzymes to maintain DNA integrity. Host commensal microbiota-derived products and IL-15 signaling, the availability of which is determined by competition with conventional T cells, drive NK cell expression of KLRG1. Greater avidity for ligand via higher cell surface concentration of Ly49H and epigenetic programs that drive a particular suite of memory genes may also converge to dictate NK cell memory precursor identity. Green font represents positive regulators of memory potential. Red font represents negative regulators of memory potential. B, Comparison of the gene expression profile of Rag2−/− and wild-type NK cells with that of MPECs and SLECs. Rag2−/− and wild-type NK cells were purified from mixed bone marrow chimeric mice and RNA-sequencing performed. Heat map shows the relative mRNA expression in Rag2−/− and wild-type NK cells of the top differentially expressed genes between MPEC and SLEC populations, as previously described (89). The transcriptional signature of Rag2−/− NK cells resembles that of SLECs whereas wild-type NK cells exhibit an MPEC-like signature.