Abstract
Aim
The proper preoperative diagnosis and management of cervical proliferative disorders presenting with multiple cysts, including minimal deviation adenocarcinoma (MDA), lobular endocervical glandular hyperplasia (LEGH), and nabothian cyst (NC), have not been fully established. We previously proposed a management protocol comprising a diagnostic approach using cytology, magnetic resonance imaging, and gastric‐type mucin and subsequent treatment. We herein evaluate the usefulness of this protocol and implications of GNAS mutations in LEGH.
Methods
The clinical courses of 94 patients with cervical multicystic lesions who visited our hospital between June 1995 and September 2014 were retrospectively analyzed. GNAS mutations were investigated in 10 LEGH, five LEGH with atypia, and two MDA cases.
Results
Of the 94 patients, the conditions of 10, 59, and 25 were clinically diagnosed as suspicious of MDA or carcinoma (S/O MDA‐Ca), suspicious of LEGH (S/O LEGH), and NC, respectively. Ten patients each with S/O MDA‐Ca and S/O LEGH underwent hysterectomy, and the correct ratio for diagnosis was 90% (18/20). Of the 42 S/O LEGH cases followed‐up for more than 12 months, three showed an increase in tumor size. After hysterectomy, two were LEGH with atypia while one was NC. The GNAS mutation was detected in two cases of LEGH with atypia, one of which showed an increase in tumor size during follow‐up.
Conclusion
The management protocol we propose herein will be useful. An increase in tumor size is important to detect potentially malignant LEGH. GNAS mutations may be involved in the tumorigenesis of potentially malignant LEGH.
Keywords: adenocarcinoma, follow‐up studies, gastric mucins, mutation, uterine cervical neoplasms
Introduction
The prevalence of image analyses, such as transvaginal ultrasonography and magnetic resonance imaging (MRI), has enhanced the detection of multicystic lesions in the uterine cervix, such as minimal deviation adenocarcinoma (MDA), lobular endocervical glandular hyperplasia (LEGH), and nabothian cyst (NC). MDA requires prompt curative surgery, whereas expectant treatments may be chosen for women suspected of having LEGH, especially for young women to preserve fertility. Therefore, a precise preoperative diagnosis and adequate management of these diseases are very important to avoid the under‐ or over‐treatment of patients.
MDA,1 formerly referred to as adenoma malignum,2 is an extremely well‐differentiated mucinous adenocarcinoma consisting of deceptively normal‐looking cervical glands. Its prognosis is poor with resistance to surgical or medical therapy and high rates of lymph node metastasis and peritoneal dissemination have been reported.3 A unique symptom of MDA is a massive watery vaginal discharge with a swollen cervix and multicystic lesions. Furthermore, MDA produces gastric‐type mucin. As cellular atypia of MDA is minimal, the cytology of the cervix is often negative.4 Difficulties are also associated with obtaining an accurate diagnosis due to limited biopsy samples. Therefore, a preoperative diagnosis of MDA is considered to be extremely difficult.
LEGH was first reported by Nucci et al. in 1999 as a benign lesion characterized by the lobular proliferation of small glands lined by endocervical mucin‐producing epithelial cells,5 which is similar to that of MDA except for the lack of nuclear atypia and stromal invasion. LEGH also exhibits similar symptoms, such as a watery discharge containing gastric‐type mucin. Therefore, a differential diagnosis between LEGH and MDA is challenging. Although LEGH was first reported as a benign disease, it has been reported as a precursor of MDA, because of frequent association between LEGH and MDA as well as its genetic characteristics.6, 7, 8, 9, 10, 11 We previously reported that some LEGH lesions were a precancerous form of MDA due to the pattern of X chromosome inactivation using the human androgen receptor (HUMARA) method.12 Therefore, the possibility of a focal association with carcinoma in LEGH needs to be considered in its management. However, proper primary clinical diagnosis and follow‐up, including a method to detect the potential malignant transformation of LEGH, have not yet been established. Moreover, when benign nabothian cysts tightly aggregate, a differential diagnosis of these lesions from LEGH or MDA is sometimes difficult.13, 14
In order to improve diagnostic accuracy, we carried out a multicenter study during 2006–2007.15 Data were collected from patients with MDA or other related diseases in 24 institutions in Japan. The clinicopathologic characteristics, gastric‐type mucin, and MRI findings of these cases were analyzed, and a management protocol was proposed for patients with cervical multicystic lesions (Fig. 1). In the present study, we retrospectively analyzed the clinical courses of 94 patients with cervical multicystic lesions managed in our hospital according to this protocol in order to evaluate its effectiveness.
Figure 1.
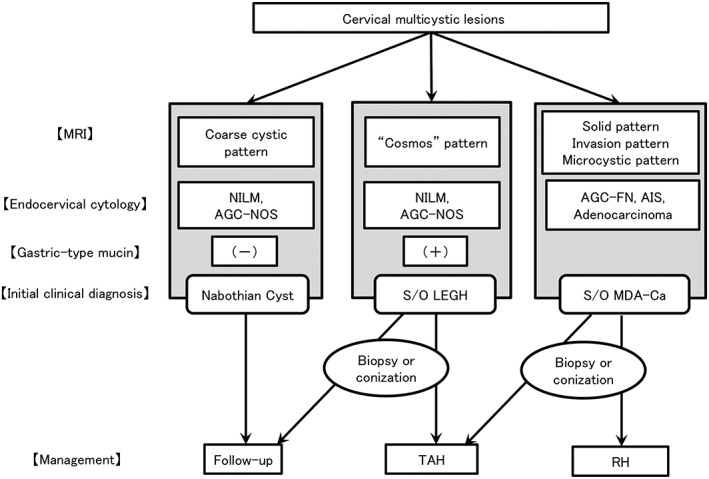
Flow chart for the diagnosis and management of cervical multicystic lesions. This figure is a modified version of our original protocol. AGC‐FN, atypical glandular cells – favor neoplastic; AGC‐NOS, atypical glandular cells – not otherwise significant; AIS, adenocarcinoma in situ; Ca, carcinoma; LEGH, lobular endocervical glandular hyperplasia; MDA, minimal deviation adenocarcinoma; MRI, magnetic resonance imaging; NILM, negative for intraepithelial lesion or malignancy; RH, radical hysterectomy; S/O, suspicion of; TAH, total abdominal hysterectomy.
A clear understanding of the molecular biology of LEGH is important for determining proper management because it may convert to malignancy. Oncogenic mutations in the GNAS gene have recently been identified as a cause of mucin‐producing neoplasms in the pancreas and gastrointestinal tract.16 The GNAS gene encodes the α‐subunit of the stimulatory guanine nucleotide‐binding protein (Gsα), which transduces signals from a G protein‐coupled receptor. GNAS has been shown to elevate intracellular cAMP levels by stimulating adenylyl cyclase,17 which provokes cellular proliferation through the protein kinase A‐ERK signal pathway.18 Previous studies have detected these mutations in glandular neoplasms in the pancreas, colon, stomach, duodenum, and appendix16, 19, 20, 21, 22, 23, 24 Matsubara et al. also identified GNAS gene mutations in 42% of LEGH.25 However, the relation among GNAS mutations, clinical courses and histological types of LEGH remains unclear. In the present study, we analyzed GNAS mutations in 17 patients whose clinical courses were available and evaluated the implications of these mutations in the management of LEGH.
Methods
Subjects
Ninety‐four women with multicystic lesions detected by transvaginal ultrasonography, MRI, or computed tomography who visited Shinshu University Hospital between June 1995 and September 2014 participated in this study. The usefulness of the protocol was retrospectively evaluated with respect to the precision of the diagnosis, result of the follow‐up, and patient outcomes.
Initial clinical diagnosis, treatment, and follow‐up
In women with multiple cervical cysts, the primary clinical diagnosis and subsequent management was provided according to our protocol15 with some modifications (Fig. 1). In this protocol, the combination of three parameters – MRI, cervical Pap smear, and gastric‐type mucin – was used for a clinical diagnosis. MRI‐T2 image findings were classified as follows: (i) solid pattern (a solid component was noted, suggesting malignancy); (ii) invasion pattern (diffuse and solid high T2‐weighted signal with an unclear margin in the cervical stroma, suggesting malignancy); (iii) cosmos pattern (diffuse or microcystic parts surrounded by medium to large cysts, suggesting LEGH); (iv) microcystic pattern (aggregation of small cysts without peripheral large cysts, suggesting LEGH or malignancy); and (v) coarse cystic pattern (irregular aggregation of medium to large cysts without a solid or microcystic component, suggesting NC). A cytological diagnosis was made according to the Bethesda system. Gastric‐type mucin in the cervical mucus was examined using a latex agglutination assay (HIK1083 kit)26 or by the detection of ‘yellow’ or ‘orange’ mucin in the cervical Pap smear.4 A clinical diagnosis and subsequent treatment were summarized as follows: the patient's condition was regarded as suspicious of MDA or adenocarcinoma (S/O MDA‐Ca) when pelvic MRI revealed a predominant solid component or invasive lesion and/or atypical glandular cells (AGC) in the Pap smear. Patients with S/O MDA‐Ca underwent immediate biopsy and curative hysterectomy. When MRI showed small cysts or solid parts surrounded by larger cysts (‘cosmos pattern’), no malignant cell, or at most, atypical glandular cells were noted in the Pap smear, and gastric‐type mucin was positive, the condition was regarded as suspicious of LEGH (S/O LEGH). Patients with S/O LEGH chose surgery (conization or hysterectomy) or follow‐up every 3–6 months. Women with coarse, multiple cysts with a clear margin, negative cytology, and gastric‐type mucin were considered to have NC, and follow‐up was recommended every 6–12 months. In patients who chose follow‐up, when MRI findings suggested malignancy, for example, an increase in lesion size or the novel detection of a solid/ invasive portion, and when the deterioration of cytology was observed, these patients ceased follow‐up and surgery was recommended. Regarding patients who visited our hospital before 2006 (11 cases), a clinical diagnosis by this protocol was made according to the patient's chart. This study was approved by the Ethics Committee of Shinshu University School of Medicine.
Mutational analysis of the GNAS gene
The formalin‐fixed paraffin‐embedded tissue sections of 10 cases of LEGH, five of LEGH with atypia, and two of MDA lesions were obtained by hysterectomy. Neoplastic glandular cells were dissected from these tissue sections using laser microdissection. Briefly, 10‐μm‐thick sections from paraffin‐embedded blocks were deparaffinized and stained by hematoxylin. The laser microdissection system PALM Micro Beam (Zeiss) was used to collect cells of interest. Genomic DNA was extracted from the dissected tissues using the QIAamp DNA Micro Kit (Qiagen). The sequence of extracted DNA was determined using the ABI310 Genetic Analyzer (Applied Biosystems) after PCR with the primer pairs directing exon 8 (GGCTTTGGTGAGATCCATTGAC , TGGCTTACTGGAAGTTGACTTTG) and 9 (GACATTCACCCCAGTCCCTCTGG, GAACAGCCAAGCCCACAGCA) of the GNAS gene, as previously reported.25
Results
The mean age of the 94 patients was 47.8 years (range: 25–77). The number of patients with a clinical diagnosis of S/O MDA‐Ca, S/O LEGH, and NC made according to our protocol (Fig. 1) was 10, 59, and 25, respectively (Table 1).
Table 1.
Patient characteristics
| Clinical diagnosis | Number of cases | Age | Watery discharge | Abnormal cytology (Gl. atypia) |
|---|---|---|---|---|
| Mean ± SD | n (%) | n (%) | ||
| S/O MDA‐Ca | 10 | 50.7 ± 12.2 | 5 (50%) | 7 (70%) |
| S/O LEGH | 59 | 46.7 ± 9.8 | 30 (50.8%) | 5 (8.5%) |
| NC | 25 | 48.9 ± 11.2 | 4 (16%) | 0 (0%) |
| Total | 94 | 47.8 ± 10.5 | 39 (41.5%) | 12 (12.8%) |
Gl. atypia, glandular atypia; NC, nabothian cyst; SD, standard deviation; S/O MDA‐Ca, suspicious of minimal deviation adenocarcinoma or carcinoma; S/O LEGH, suspicious of lobular endocervical glandular hyperplasia.
Initial clinical diagnosis and management of each disease group
Patients clinically diagnosed according to our protocol and their clinical courses are summarized in Figure 2 and Table 2. All 10 patients with S/O MDA‐Ca underwent hysterectomy (radical hysterectomy, n = 6; total abdominal hysterectomy [TAH], n = 4), and a pathological diagnosis was finally determined. Seven out of the 10 S/O MDA‐Ca cases were malignant. On MRI, four of the seven malignant cases had a predominant solid component (e.g., Case 5, in Fig. 3a,b) while two had a partial solid component (e.g., Case 1, in Fig. 3c,d). Two cases (Case 8, 10) of LEGH did not show a cosmos pattern, but showed a microcystic pattern. One case (Case 9) showed invasive findings on MRI, but was LEGH with atypia and mucinous adenoma. Cytologically, of the seven malignant cases, two had adenocarcinoma and five had AGC. Five cases had gastric‐type mucin.
Figure 2.
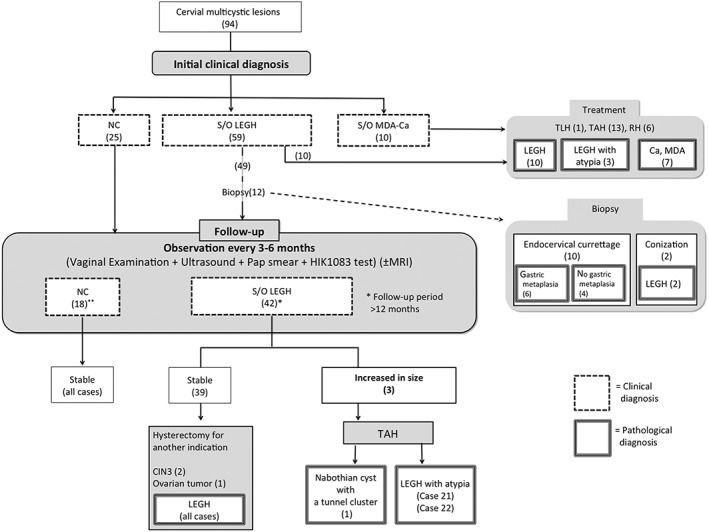
Summary of clinical courses of patients with multicystic lesions of the uterine cervix, managed according to our protocol. Ca, carcinoma; LEGH, lobular endocervical glandular hyperplasia; MDA, minimal deviation adenocarcinoma; NC, nabothian cyst; NILM, negative for intraepithelial lesion or malignancy; RH, radical hysterectomy; S/O, suspicion of; TAH, total abdominal hysterectomy; TLH, total laparoscopic hysterectomy.
Table 2.
Clinicopathologic summary of patients who underwent hysterectomy
| Clinical diagnosis | Case | MRI finding | Cytology | HIK test | Cervical biopsy | Mode of surgery | Pathological diagnosis | LM / LVSI | Ovarian‐meta | Stage | F/U period (months) | Status at last F/U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S/O MDA‐Ca | 1 | Solid and invasion pattern | Adenoca. | NA | Adenoca. | RH, BSO, PLN | Serous adenoca, LEGH | − | − | IB1 | 68 | Alive |
| 2 | Solid and invasion pattern | AGC‐FN | + | Adenoca. | RH, BSO, PLN, PAN | GAS, MDA, LEGH with atypia, LEGH | + | − | IIB | 33 | Alive | |
| 3 | Solid pattern | AGC‐FN | + | Adenoca. | RH, BSO, PLN | GAS, Endocervical type muc. adenoca. | + | + | IIA1 | 19 | DOD | |
| 4 | Solid pattern | AGC‐FN | NA | MDA | TAH, BSO, PLN, PAN | GAS, MDA | + | + | IB2 | 5 | DOD | |
| 5 | Solid pattern | Adenoca. | + | Adenoca. | RH, BSO, PLN. PAN | GAS, MDA, LEGH | + | − | IB2 | 3 | Alive | |
| 6 | Microcystic pattern | AGC‐FN | + | Adenoca. | TAH, BSO | GAS, LEGH | − | − | IIA1 | 4 | Alive | |
| 7 | Solid pattern | AGC‐NOS | NA | NA | RH, BSO, PLN | MDA | − | − | IB1 | 24 | DOD | |
| 8 | Microcystic pattern | AGC‐FN | + | NA | TAH | LEGH with atypia | − | − | − | 63 | Alive | |
| 9 | Solid and invasion pattern | NILM | − | Normal | TAH, BSO | LEGH with atypia + mucinous adenoma | − | − | − | 20 | Alive | |
| 10 | Microcystic pattern | NILM | NA | Normal | RH, BSO, PLN | LEGH | − | − | − | 131 | Alive | |
| S/O LEGH | 11 | Cosmos pattern | NILM | + | NA | TAH | LEGH with atypia | − | − | − | 32 | Alive |
| 12 | Cosmos pattern | AGC‐NOS | + | NA | TAH | LEGH | − | − | − | 33 | Alive | |
| 13 | Coarse cystic pattern | AGC‐NOS | + | NA | TAH | LEGH | − | − | − | 85 | Alive | |
| 14 | Microcystic pattern | AGC‐NOS | + | LEGH | TAH, BSO | LEGH | − | − | − | 33 | DOO | |
| 15 | Cosmos pattern | AGC‐NOS | + | NA | TAH | LEGH | − | − | − | 21 | Alive | |
| 16 | Cosmos pattern | NILM | + | Normal | TAH | LEGH | − | − | − | 61 | Alive | |
| 17 | Cosmos pattern | NILM | + | LEGH | TAH, BSO | LEGH | − | − | − | 12 | Alive | |
| 18 | Cosmos pattern | AGC‐NOS | + | NA | TLH, BSO | LEGH | − | − | − | 17 | Alive | |
| 19 | Coarse cystic pattern | NILM | + | NA | TAH | LEGH | − | − | − | 136 | Alive | |
| 20 | Coarse cystic pattern | NILM | + | Normal | TAH, BSO | LEGH | − | − | − | 122 | Alive |
Adenoca., adenocarcinoma; AGC‐FN: atypical glandular cells‐favor neoplastic, AGC‐NOS: atypical glandular cells‐not otherwise significant, BSO: bilateral salpingo‐oophorectomy, DOD: dead of disease, DOO: dead of other causes; F/U, follow‐up; GAS: gastric‐type adenocarcinoma, LEGH: lobular endocervical glandular hyperplasia, LM: lymph node metastasis, LVSI: lymphovascular space invasion, MDA, minimal deviation adenocarcinoma; MRI, magnetic resonance imaging; muc. adenoca., mucinous adenocarcinoma; NA, not available; NILM, negative for intraepithelial lesion or malignancy; PAN: para‐aortic lymphadenectomy, PLN: pelvic lymphadenectomy, RH: radical hysterectomy, TAH: total abdominal hysterectomy, TLH: total laparoscopic hysterectomy; S/O MDA‐Ca, suspicious of minimal deviation adenocarcinoma or carcinoma; S/O LEGH, suspicious of lobular endocervical glandular hyperplasia.
Figure 3.
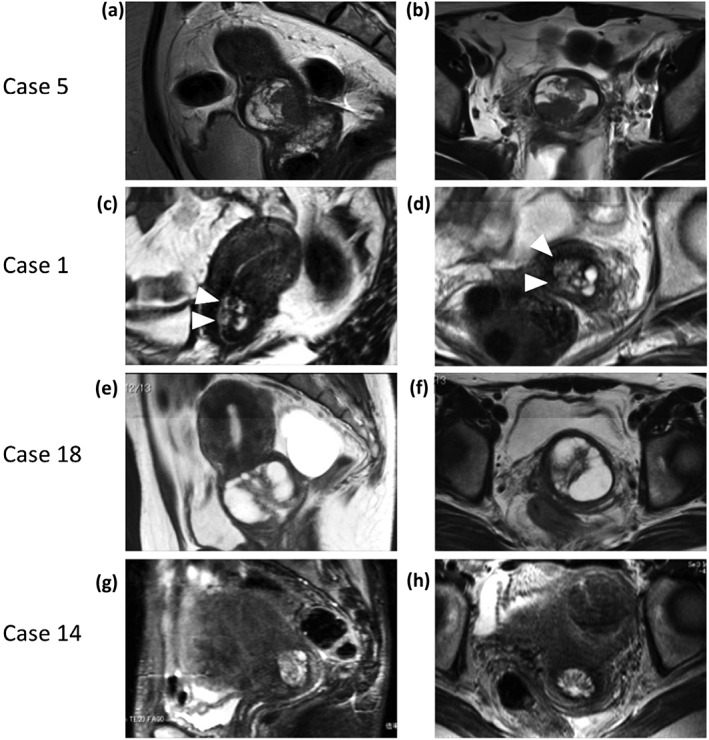
Magnetic resonance imaging findings of patients who underwent hysterectomy, as shown in Table 2. (a,b) A typical solid pattern was observed in case 5. (c,d) A combination of solid pattern and invasion pattern. Microcystic and solid components (arrows) existed in the lateral portion of the cervix, suggesting stromal invasion, as observed in Case 1. (e,f) A typical cosmos pattern observed in Case 18 (i.e., small cysts or solid parts were surrounded by larger cysts). (g,h) A microcystic pattern: the aggregation of micro cysts, with the absence of large surrounding cysts or signs of invasion in Case 14.
Ten out of 59 patients with S/O LEGH underwent hysterectomy (total laparoscopic hysterectomy, n = 1; TAH, n = 9, Table 2). The histological diagnosis of these patients was LEGH in nine and LEGH with atypia in one, with six showing a cosmos pattern on MRI (e.g., Case 18 in Fig. 3e,f), and the others a coarse cystic pattern (three cases) and a microcystic pattern (Case 14, in Figs. 3g,h). Cytologically, five cases each showed negative for intraepithelial lesion or malignancy (NILM) or AGC – not otherwise significant (NOS). All 10 patients had gastric‐type mucin in cervical mucus. Twelve patients out of 49 S/O LEGH patients who did not undergo hysterectomy underwent biopsy (conization, n = 2; endocervical curettage, n = 10).
Collectively, these results demonstrated a high diagnostic accuracy for LEGH and MDA/carcinoma: the correct ratio for diagnosis was 90% (19/20).
Results of the follow‐up patients
After the initial clinical diagnosis, 25 patients with NC and 49 patients with S/O LEGH chose regular follow‐ups with informed consent (Fig. 2). Of these patients, 18 with NC and 42 with S/O LEGH were followed‐up for 12 months or more. No change in MRI or cytology was observed in any of the 18 NC patients. Of the 42 patients with S/O LEGH, 39 showed no change in lesion size on MRI, whereas three showed an increased tumor diameter. Of the 39 lesion‐stable patients, three underwent hysterectomy due to other diseases (CIN3, two cases; ovarian cyst, one case). The pathological findings in the cervix of these patients were LEGH. The three patients with an increased lesion size underwent hysterectomy. Histologically, one patient had NC, whereas the remaining two had LEGH with atypia (Cases 21 and 22). Gastric‐type mucin was constantly positive in most follow‐up cases.
Clinicopathology of two patients with S/O LEGH with an increased lesion size during follow‐up
Case 21 was a 55‐year‐old woman (gravida 1, para 1, menopause at 53 years old) with a watery vaginal discharge and irregular bleeding. Transvaginal ultrasonography revealed a multicystic lesion with AGC‐NOS cytology and positive gastric type mucin by the HIK1083 kit. MRI showed a 15 × 10 × 8‐mm multicystic lesion indicating a cosmos pattern (Fig. 4a). The clinical diagnosis was S/O LEGH. As the resolution of the first MRI was poor, MRI was again performed after 3 months. The second MRI revealed that the size of the lesion had increased to 21 × 21 × 14 mm with diffuse small cysts (Fig. 4b). Due to suspected MDA, TAH was performed. The pathological diagnosis was LEGH with atypia (Fig. 4c). No recurrence was recorded 51 months after surgery.
Figure 4.
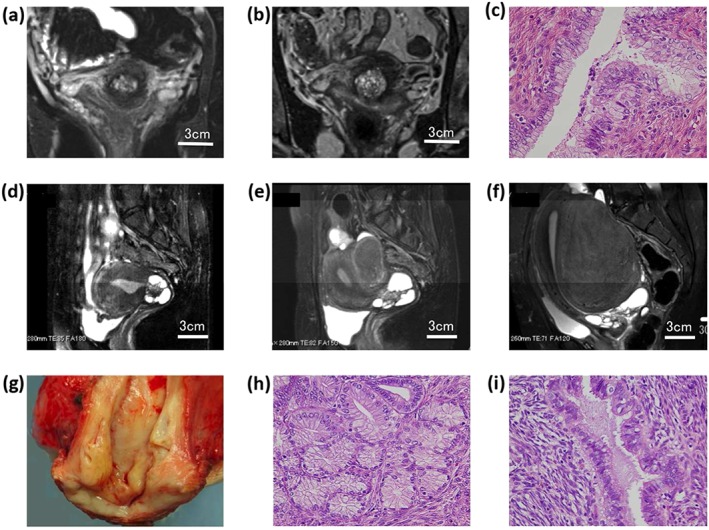
Magnetic resonance imaging (MRI) and pathologic findings of two cases with increased lesion sizes during the follow‐up. (a–c) Case 21. (a) MRI showed the lesion size to be 15 × 10 × 8 mm at the first visit. (b) The lesion increased to 21 × 21 × 14 mm with more small cysts. (c) Hysterectomy specimen histologically showed lobular endocervical glandular hyperplasia (LEGH) with atypia. (d–i) Case 22. (d) The lesion size was 39 × 33 × 33 mm with a typical cosmos pattern at the first visit. (e) The lesion size increased 4 years later. (f) The lesion increased 66 × 45 × 37 mm 12 years after the first MRI. (g) A hysterectomy specimen showing a watery discharge. (h,i) LEGH with atypia was noted.
Case 22 was a 33‐year‐old woman (gravida 0) with dysmenorrhea. MRI revealed a 39 × 33 × 33‐mm multicystic lesion with a typical cosmos pattern in the cervix and adenomyosis in the uterine corpus. The Pap smear was NILM and the HIK1083 test was negative. The patient was followed‐up as an S/O LEGH case. At the age of 37, she developed a watery discharge. The Pap smear was AGC‐NOS and HIK1083 became positive at the age of 40. MRI scans were repeatedly performed every 1–3 years, which revealed that the lesion size had gradually increased. By the age of 45, the lesion had reached 66 × 45 × 37 mm (Fig. 4d–f). The patient underwent diagnostic conization, which revealed LEGH with atypia. Hysterectomy was performed and the diagnosis was LEGH with atypia (Fig. 4g–i). No recurrence was recorded 22 months after surgery.
Mutational analysis of the GNAS gene
The results of a mutational analysis are shown in Table 3. Point mutations leading to the amino acid changes p.R201H or p.R201C were observed in two cases of LEGH with atypia (Case 9 in Tables 2 and 3, and Case 21 in Table 3). In case 9, a mutation was not detected in the co‐existing LEGH part. Case 21 showed an increase in lesion size during the follow‐up, as discussed above.
Table 3.
Mutational analysis of the GNAS gene
| Case | Age (years) | Clinical signs | HIK test | Increased lesion size | Histology of the dissected area | GNAS gene mutation | Peptide alteration |
|---|---|---|---|---|---|---|---|
| 2 | 49 | Watery discharge, | + | No | MDA | WT | WT |
| Vaginal bleeding | LEGH with atypia | WT | WT | ||||
| LEGH | WT | WT | |||||
| 7 | 42 | Vaginal bleeding | NA | No | MDA | WT | WT |
| 8 | 34 | Watery discharge | + | No | LEGH with atypia | WT | WT |
| 9 | 57 | Cervical cyst | − | No | LEGH with atypia | c.601 C > T | p.R201C |
| Vaginal bleeding | LEGH | WT | WT | ||||
| 21 | 54 | Watery discharge | + | Yes | LEGH with atypia | c.602 G > A | p.R201H |
| 22 | 33 | Watery discharge | + | Yes | LEGH with atypia | WT | WT |
| 12 | 42 | Watery discharge | + | No | LEGH | WT | WT |
| 13 | 42 | Cervical cyst | + | No | LEGH | WT | WT |
| 14 | 49 | Cervical cyst | + | No | LEGH | WT | WT |
| 16 | 46 | Watery discharge | + | No | LEGH | WT | WT |
| 17 | 40 | Watery discharge | + | No | LEGH | WT | WT |
| 19 | 68 | Vaginal bleeding | + | No | LEGH | WT | WT |
| 10 | 47 | Watery discharge | NA | No | LEGH | WT | WT |
| 23 | 48 | Uterine prolapse | NA | No | LEGH | WT | WT |
In Case 2 and 9, each lesions (MDA, LEGH with atypia and LEGH) were separately collected and used for the analysis. LEGH, lobular endocervical glandular hyperplasia; MDA, minimal deviation adenocarcinoma; NA, not available; WT, wild type.
Discussion
The present study demonstrates that the clinical diagnoses made by our management protocol were largely consistent with the final pathologic diagnoses of surgically extirpated tissues, which indicates that our protocol is accurate and useful for practical use. In the diagnosis of malignant diseases, the detection of a solid component or invasive findings on MRI and/or abnormal cytology appears to be crucial. Cytologic atypia was observed in three (cases 3–5) out of four cases with a predominant solid component pattern on MRI. The diagnoses of these three cases were relatively simple; however, the diagnosis became more difficult when the cytology showed AGC‐NOS with a predominantly solid pattern on MRI, as observed in a case of MDA (case 7). In this case, MRI may be more valuable for a diagnosis than a Pap smear because MDA sometimes shows only normal or minimal atypia on cytology. A partial solid component on MRI was also an important sign for malignancy. Case 3 was histologically confirmed as gastric‐type adenocarcinoma (GAS), which was recently established and is reported to have a poor prognosis.27 Therefore, the detection of gastric‐type mucin by the HIK1083 kit may assist in the selection of appropriate management, including radical surgery. Although histological diagnosis of Case 10 was LEGH, the clinical diagnosis was MDA. The difficulty in achieving an accurate preoperative diagnosis in this case may have been due to the lack of a ‘cosmos’ sign. Two cases of LEGH with atypia (cases 8 and 9) showed an intermediate pattern; either MRI or cytology‐suggested malignancy.
Of the 10 cases with S/O LEGH, nine were LEGH and one was LEGH with atypia, with the correct ratio being 90%. LEGH was clinically characterized by a cosmos pattern on MRI and no or slight cytologic atypia and gastric‐type mucin. In this regard, the diagnosis of six cases (cases 11, 12, 15–18) appeared to be simple. The detection of gastric‐type mucin had important diagnostic impact in the remaining four cases. However, we were unable to identify any specific findings to extract LEGH with atypia (case 11) from other ‘ordinary’ LEGH cases. It may be noteworthy that there was one case of atypical LEGH among the ordinary LEGH cases (i.e., 10%), with the ratio being similar to that of our previous study in which approximately 5–10% of clinically diagnosed LEGH cases had malignant lesions.15 Therefore, our protocol is useful for the clinical diagnosis for cervical multicystic diseases.
Of the 49 patients with S/O LEGH who did not undergo hysterectomy, 10 and two underwent cervical curettage and conization, respectively. Six out of the 10 patients who underwent cervical curettage showed gastric metaplasia of the cervical glands. Gastric metaplasia is considered to be the early stage of the development of LEGH,6 and this finding may indirectly suggest the presence of LEGH; however, none of the specimens from cervical curettage provided definite evidence of LEGH containing the lobular proliferation of small glands. Therefore, conization may be preferred for cases in which other findings are not informative.
In the present study, 42 patients with S/O LEGH chose to undergo follow‐up. Although LEGH was initially proposed to be a benign disease, subsequent studies have revealed the possibility of LEGH as a precursor of carcinoma.11 However, the clinical signs indicating the malignant transformation of LEGH currently remain unclear. Therefore, we tentatively determined changes in MRI findings, such as increases in size or the detection of solid parts, as well as the deterioration of cytology as the signs of malignant changes. When we detected these findings, we recommended surgical management. According to the follow‐up policy, three patients showed an increased lesion diameter and subsequently underwent hysterectomy. Of these three patients, two had LEGH with atypia. Sugihara et al. reported a case that was initially diagnosed as LEGH, but histologically diagnosed as MDA after a 2‐year follow‐up, which revealed an increase in the size of the lesion (from 21 mm to 31 mm in diameter), and the deterioration of the Pap smear.28 They concluded that the deterioration of Pap smear findings and increase in lesion size were important signs of the malignant progression of LEGH lesions to MDA. Therefore, we consider changes in MRI findings and cytology as the hallmarks of malignant transformation. The number of young patients in whom fertility needs to be preserved has sharply increased recently, and most young patients do not choose hysterectomy. Therefore, these parameters are very important in the management of young patients suspected of having LEGH.
There are several reports suggesting that LEGH is a precursor lesion of GAS, including MDA, based on the fact that LEGH is frequently associated with MDA and/or GAS.6, 7, 8, 9, 10, 29 More recently, atypical LEGH was described as LEGH with atypical features, such as nuclear atypia and papillary architecture.11 Although the definitive morphologic classification and its tumorigenic implication have not been fully established, this entity may be the possible link between LEGH and GAS. Ohta et al. reported a case with sequential lesion of LEGH, LEGH with atypia, and MDA,30 supporting this concept. In addition, the tumor suppressor STK11 gene mutation has been of interest since the patients of Peutz‐Jeghers syndrome harboring its mutation have been reported to associate with LEGH, MDA, and adenocarcinoma.31 Kuragaki et al. reported that 55% of MDA cases had a mutation in the STK11 gene.32 We also reported that STK11 may be involved in the progression of LEGH to MDA, according to a case containing mixed lesion of LEGH and MDA with the same clonality, whereas STK11 gene mutation was observed only in MDA lesion.12 In the present study, GNAS gene mutations were detected in two (14.3%) out of 14 cases of LEGH. Matsubara et al. identified this mutation in eight (42%) out of 19 cases of LEGH,25 which was higher than in our study. The reason for this difference currently remains unclear, but our tissue sampling procedure using laser microdissection may be more precise than their method using sterilized toothpicks. Two patients with GNAS mutation in our study had atypical glandular cells, one of which also had an increased lesion size. Although the oncogenic potential of GNAS mutations has not yet been fully evaluated, GNAS mutations are known to elevate cAMP levels in the cytosol, leading to an active metabolic status. Therefore, these findings may indicate the involvement of these mutations in the process of activating or destabilizing the biological behavior of tumor cells, and thus may play a role in the conversion of LEGH. The tumorigenic mechanism of LEGH has not yet been examined. Further studies are needed to clarify the significance of GNAS mutations in the tumorigenic process of LEGH and the possibility of clinical applications.
In conclusion, the management protocol that we proposed for the clinical diagnosis and treatment of cervical multicystic lesions is useful. Furthermore, an increase in tumor size and deteriorating cytology are important parameters for detecting potentially malignant LEGH during follow‐up. Further investigations are needed to evaluate the impact of GNAS mutations in tumorigenesis and clinical applications.
Disclosure
The authors have no potential conflicts of interest to disclose.
Acknowledgments
This work was supported by JSPS KAKENHI (no. 15 K10712, 25 670 699).
Ando, H. , Miyamoto, T. , Kashima, H. , Takatsu, A. , Ishii, K. , Fujinaga, Y. , and Shiozawa, T. (2016) Usefulness of a management protocol for patients with cervical multicystic lesions: A retrospective analysis of 94 cases and the significance of GNAS mutation. J. Obstet. Gynaecol. Res., 42: 1588–1598. doi: 10.1111/jog.13083.
References
- 1. Silverberg SG, Hurt WG. Minimal deviation adenocarcinoma (“adenoma malignum”) of the cervix: A reappraisal. Am J Obstet Gynecol 1975; 121: 971–975. [DOI] [PubMed] [Google Scholar]
- 2. Gusserow ALS . Ueber sarcome des uterus. Arch Gynakol 1870; 1: 240–251. [Google Scholar]
- 3. Wright TC, Ferenczy AKJ. Blaustein's Pathology of the Female Genital Tract, 6th edn. New York: Springer‐Verlag, 2011. [Google Scholar]
- 4. Ishii K, Katsuyama T, Ota H et al. Cytologic and cytochemical features of adenoma malignum of the uterine cervix. Cancer 1999; 87: 245–253. [DOI] [PubMed] [Google Scholar]
- 5. Nucci MR, Clement PB, Young RH. Lobular endocervical glandular hyperplasia, not otherwise specified: A clinicopathologic analysis of thirteen cases of a distinctive pseudoneoplastic lesion and comparison with fourteen cases of adenoma malignum. Am J Surg Pathol 1999; 23: 886–891. [DOI] [PubMed] [Google Scholar]
- 6. Mikami Y, Kiyokawa T, Hata S et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: A possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and “adenoma malignum”. Mod Pathol 2004; 17: 962–972. [DOI] [PubMed] [Google Scholar]
- 7. Kondo T, Hashi A, Murata S et al. Endocervical adenocarcinomas associated with lobular endocervical glandular hyperplasia: A report of four cases with histochemical and immunohistochemical analyses. Mod Pathol 2005; 18: 1199–1210. [DOI] [PubMed] [Google Scholar]
- 8. Nishio S, Tsuda H, Fujiyoshi N et al. Clinicopathological significance of cervical adenocarcinoma associated with lobular endocervical glandular hyperplasia. Pathol Res Pract 2009; 205: 331–337. [DOI] [PubMed] [Google Scholar]
- 9. Liao SY, Rodgers WH, Kauderer J et al. Endocervical glandular neoplasia associated with lobular endocervical glandular hyperplasia is HPV‐independent and correlates with carbonic anhydrase‐IX expression: A Gynaecological Oncology Group Study. Br J Cancer 2013; 108: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nara M, Hashi A, Murata S‐I et al. Lobular endocervical glandular hyperplasia as a presumed precursor of cervical adenocarcinoma independent of human papillomavirus infection. Gynecol Oncol 2007; 106: 289–298. [DOI] [PubMed] [Google Scholar]
- 11. Mikami Y, McCluggage WG. Endocervical glandular lesions exhibiting gastric differentiation: An emerging spectrum of benign, premalignant, and malignant lesions. Adv Anat Pathol 2013; 20: 227–237. [DOI] [PubMed] [Google Scholar]
- 12. Takatsu A, Miyamoto T, Fuseya C et al. Clonality analysis suggests that STK11 gene mutations are involved in progression of lobular endocervical glandular hyperplasia (LEGH) to minimal deviation adenocarcinoma (MDA). Virchows Arch 2013; 462: 645–651. [DOI] [PubMed] [Google Scholar]
- 13. Clement PB, Young RH. Deep nabothian cysts of the uterine cervix. A possible source of confusion with minimal‐deviation adenocarcinoma (adenoma malignum). Int J Gynecol Pathol 1989; 8: 340–348. [DOI] [PubMed] [Google Scholar]
- 14. Oguri H, Maeda N, Izumiya C, Kusume T, Yamamoto Y, Fukaya T. MRI of endocervical glandular disorders: Three cases of a deep nabothian cyst and three cases of a minimal‐deviation adenocarcinoma. Magn Reson Imaging 2004; 22: 1333–1337. [DOI] [PubMed] [Google Scholar]
- 15. Takatsu A, Shiozawa T, Miyamoto T et al. Preoperative differential diagnosis of minimal deviation adenocarcinoma and lobular endocervical glandular hyperplasia of the uterine cervix: A multicenter study of clinicopathology and magnetic resonance imaging findings. Int J Gynecol Cancer 2011; 21: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 16. Furukawa T, Kuboki Y, Tanji E et al. Whole‐exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011; 1: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 1989; 340: 692–696. [DOI] [PubMed] [Google Scholar]
- 18. Stork PJS, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 2002; 12: 258–266. [DOI] [PubMed] [Google Scholar]
- 19. Yamada M, Sekine S, Ogawa R et al. Frequent activating GNAS mutations in villous adenoma of the colorectum. J Pathol 2012; 228: 113–118. [DOI] [PubMed] [Google Scholar]
- 20. Matsubara A, Sekine S, Kushima R et al. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol 2013; 229: 579–587. [DOI] [PubMed] [Google Scholar]
- 21. Nishikawa G, Sekine S, Ogawa R et al. Frequent GNAS mutations in low‐grade appendiceal mucinous neoplasms. Br J Cancer 2013; 108: 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsubara A, Ogawa R, Suzuki H et al. Activating GNAS and KRAS mutations in gastric foveolar metaplasia, gastric heterotopia, and adenocarcinoma of the duodenum. Br J Cancer 2015; 112: 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zauber P, Marotta S, Sabbath‐Solitare M. GNAS mutations are associated with mucin production in low‐grade appendiceal mucinous neoplasms, villous adenomas, and carcinomas. Hum Pathol 2015; 46: 339. [DOI] [PubMed] [Google Scholar]
- 24. Singhi AD, Davison JM, Choudry HA et al. GNAS is frequently mutated in both low‐grade and high‐grade disseminated appendiceal mucinous neoplasms but does not affect survival. Hum Pathol 2014; 45: 1737–1743.1 [DOI] [PubMed] [Google Scholar]
- 25. Matsubara A, Sekine S, Ogawa R et al. Lobular endocervical glandular hyperplasia is a neoplastic entity with frequent activating GNAS mutations. Am J Surg Pathol 2014; 38: 370–376. [DOI] [PubMed] [Google Scholar]
- 26. Ishii K, Kumagai T, Tozuka M et al. A new diagnostic method for adenoma malignum and related lesions: Latex agglutination test with a new monoclonal antibody, HIK1083. Clin Chim Acta 2001; 312: 231–233. [DOI] [PubMed] [Google Scholar]
- 27. Kojima A, Mikami Y, Sudo T et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 2007; 31: 664–672. [DOI] [PubMed] [Google Scholar]
- 28. Sugihara T, Nakagawa S, Sasajima Y, Matsumoto Y, Takeshita S, Ayabe T. Case of minimal deviation adenocarcinoma: Possible clinical link to lobular endocervical glandular hyperplasia as its origin. J Obstet Gynaecol Res 2015; 41: 483–487. [DOI] [PubMed] [Google Scholar]
- 29. Kawauchi S, Kusuda T, Liu X‐P et al. Is lobular endocervical glandular hyperplasia a cancerous precursor of minimal deviation adenocarcinoma? A comparative molecular‐genetic and immunohistochemical study. Am J Surg Pathol 2008; 32: 1807. [DOI] [PubMed] [Google Scholar]
- 30. Ohta Y, Suzuki T, Hamatani S, Shiokawa A, Kushima M, Ota H. Lobular endocervical glandular hyperplasia might become a precursor of adenocarcinoma with pyloric gland features. Pathol Res Pract 2008; 204: 683–687. [DOI] [PubMed] [Google Scholar]
- 31. Banno K, Kisu I, Yanokura M et al. Hereditary gynecological tumors associated with Peutz‐Jeghers syndrome (Review). Oncol Lett 2013; 6: 1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuragaki C, Enomoto T, Ueno Y et al. Mutations in the STK11 gene characterize minimal deviation adenocarcinoma of the uterine cervix. Lab Invest 2003; 83: 35–45. [DOI] [PubMed] [Google Scholar]


