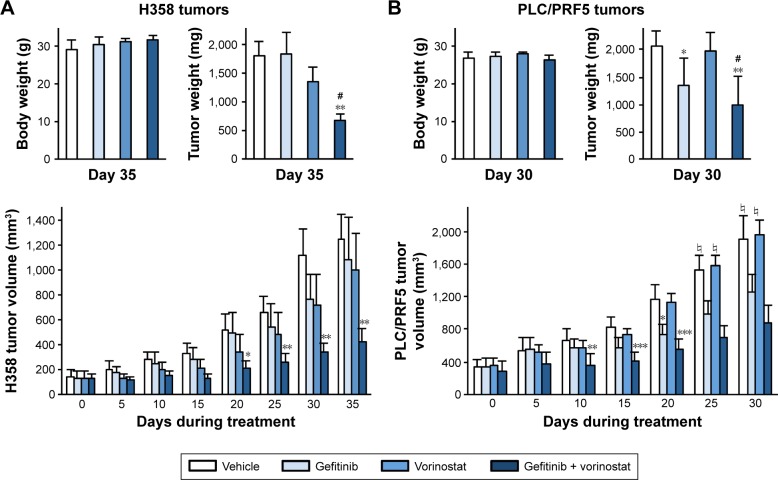
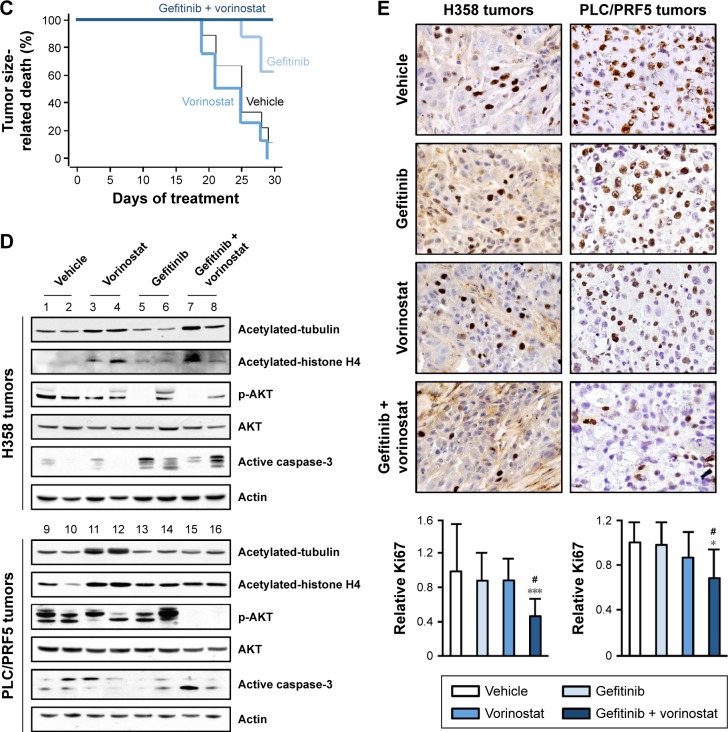
Figure 4.
Effects of the gefitinib and vorinostat combination on the growth of H358 or PLC/PRF5 xenografts.
Notes: H358 and PLC/PRF5 cells were grown as subcutaneous tumor xenografts in nude mice. After the tumors were established, the mice were treated with vehicle, gefitinib (5 mg/kg H358 or 50 mg/kg PLC/PRF5), vorinostat (100 mg/kg), or both for 30–35 days. (A, B) The means of body weight and tumor weight at sacrifice and the means of tumor volume ± SEM at the given time points in H358 (A) or PLC/PRF5 (B) xenograft models. *P<0.05; **P<0.01; ***P<0.001 compared to the control group; #P>0.05 compared to the single treatment groups. Treatment groups included 8–11 mice, except for the PLC/PRF5 xenograft model in which the vehicle and vorinostat-treated groups included only four to six mice (♮) because of euthanasia due to tumor size. (C) Tumor size-related death was calculated from the date of the beginning of treatment to the last day of the experiment or tumor size-related death for the PLC/PRF5 tumor-bearing mice. P log-rank =0.0095. (D) Effect of gefitinib and vorinostat on acetylated-tubulin, acetylated-histone H4, p-AKT and active caspase-3 in H358- (tumors 1–8), and PLC/PRF5-xenograft tumors (tumors 9–16) assessed by Western blotting (two mice per condition). (E) Ki67 nuclear protein detected through immunostaining on frozen tumor sections from mice that were treated as indicated. The Ki67 level was determined after counting positive-stained cells in ten randomly selected fields per slide in the tumor sections, and the results are expressed as the mean ± standard deviation and as the rate of staining in the vehicle-treated group (three to six mice per group). *P<0.05; ***P<0.001 compared to the control group; #P>0.05 compared to the single treatment groups.