Abstract
Drug addiction is a chronic brain disease and drugs of abuse cause long lasting neuroadaptations. Addiction is characterized by the loss of control over drug use despite harmful consequences, and high rates of relapse even after long periods of abstinence. Neurotrophic factors (NTFs) are well known for their actions on neuronal survival in the peripheral nervous system. Moreover, NTFs have been shown to be involved in synaptic plasticity in the brain. Brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) are two of the most studied NTFs and both of them have been reported to increase craving when administered into the mesocorticolimbic dopaminergic system after drug self-administration. Here we review recent data on BDNF and GDNF functions in addiction-related behavior and discuss them in relation to previous findings. Finally, we give an insight into how new technologies could aid in further elucidating the role of these factors in drug addiction.
Introduction
Drug addiction is a complex brain disorder and has a variety of causes, including genetic and environmental influences, as well as drug-induced changes in the brain (Kreek et al., 2005). Addictive drugs can alter behavior and casual use of these substances may progress to addiction. The risks for transition from casual to compulsive patterns of drug use are specific to the type of compound involved (Wagner and Anthony, 2002), and the reasons underlying this vulnerability are not well understood. Developed drug addiction is characterized by compulsive drug use, despite adverse consequences, and relapse even after long periods of withdrawal (Hunt et al., 1971; Wikler, 1973). Studies on laboratory animals have shown that activation of the mesocorticolimbic dopamine pathway in the brain has a central role in the rewarding effects of drugs of abuse and in the learning process required to form connections between contextual stimuli and rewarding or aversive events (Spanagel and Weiss, 1999). More specifically, the mesocorticolimbic dopamine pathway (Ungerstedt, 1971) originating from the VTA (ventral tegmental area) and projecting into the NAc (nucleus accumbens) and PFC (prefrontal cortex) mediates the drug reward (Wise and Rompre, 1989) and also drug-seeking relapse (Shalev et al., 2002).
During repeated drug administration neuroadaptive processes occur in the mesocorticolimbic dopamine pathway and in the glutamatergic corticolimbic circuitry. Neuroadaptation and changes in synaptic plasticity in the neural circuits mediating learning and memory of drug-induced behavior cause long-term changes in the drug-seeking behavior, leading to compulsive drug seeking and long-term vulnerability to relapse (Nestler, 2001). Because of their involvement in the regulation of activity-dependent neuronal function and synaptic plasticity, neurotrophic factors (NTFs), such as brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF), have been of interest in addiction-related research. During the past three decades studies on both how abused drugs affect the expression of NTFs, as well as how the manipulation of NTF levels can modify drug-seeking behavior have been performed (Ghitza et al., 2010).
In this review we focus on the relation between BDNF/GDNF and addiction-related behavior and neurobiology. We acknowledge previous reviews on the topic (Bolanos et al., 2005; Carnicella and Ron, 2009; Niwa et al., 2007; Pierce and Bari, 2001; Ron and Janak, 2005; Russo et al., 2009). Because of already existing extensive review of the literature on drug reward and relapse (Ghitza et al., 2010), the main focus here is the literature published in the last five years and we discuss these findings in relation to previous ones from the view of specific NTFs and drugs of abuse. We also review studies on microRNAs and endogenous antisense transcripts related to NTFs and drugs of abuse. Finally, we focus on NTFs and novel tools that could advance our knowledge in the field.
Brain regions involved in addiction-related behavior
Dopamine neurons in the ventral midbrain project to striatal, limbic, and cortical areas and form neuronal networks that regulate complex behaviors and emotions, and control voluntary motion (Liss and Roeper, 2008). A progressive age-related degeneration of mesostriatal dopamine neurons in the substantia nigra causes major motor symptoms of Parkinson’s disease (PD) (Moore et al., 2005), whereas mesocorticolimbic dopamine neurons in the VTA are essential for processing of reward-related stimuli. The mesocorticolimbic dopamine pathway originating from VTA project to the central amygdala, NAc and medial prefrontal cortex. Dopamine neurons in the VTA are targeted by drugs of abuse that elicit their rewarding effects by increasing dopamine neurotransmission as well as interacting with glutamatergic corticolimbic circuitry in a context-dependent manner (Kauer and Malenka, 2007; Luscher and Malenka, 2011; Mameli and Luscher, 2011). A single dose of an addictive drug, such as cocaine, morphine or benzodiazepines, causes adaptations at glutamate synapses on the VTA dopamine neurons within 24h after administration in mice (Heikkinen et al., 2009; Saal et al., 2003; Tan et al., 2010; Ungless et al., 2001). The drug-induced synaptic plasticity in dopamine neurons in the VTA may represent an early step in the cascade of cellular modifications leading to addiction-related behaviors.
BDNF, GDNF and dopamine
NTFs are secreted proteins that regulate differentiation and migration of neuroprogenitor cells, promote neuronal survival and modulate the number of neurons in the developing peripheral nervous system (Airaksinen and Saarma, 2002). While these processes have been demonstrated in the peripheral nervous system, surprisingly little is known about the importance of secreted NTFs for the regulation of neuronal numbers and synaptic connections in the central nervous system (CNS) in development throughout adulthood. Since the properties of NTFs give them therapeutic potential for neurological disorders, they have been extensively studied in the field of experimental neurology, and applied in animal models of neurodegeneration, e.g. Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Alzheimer’s disease (AD) (Airavaara et al., 2012; Henriques et al., 2010; Schulte-Herbruggen et al., 2008). In these models, both GDNF and BDNF, as well as several other NTFs, protect neurons from toxins and injury. Despite this, the role of endogenous NTFs in the regulation of neuronal function and survival in the adult brain still remains largely unknown.
BDNF, together with nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4), belongs to the neurotrophin family, and signals via transmembrane tyrosine kinase Trk receptors (TrkA, TrkB, TrkC) and the p75 neurotrophin receptor (Chao, 2003). Binding of the NTF ligand to Trk receptors induces receptor dimerization and autophosphorylation, which in turn activates intracellular signaling pathways, including PI3K/Akt, MEK/MAPK, and PLCγ cascades that mediate neuronal differentiation and survival, and neurite outgrowth. While the p75 neurotrophin receptor can promote activation of the Trk receptors by increasing receptor affinity and selectivity for the neurotrophins, activation of the p75 receptor by immature pro-forms of the neurotrophins seems to shape the nervous system by mediating cell death. The GDNF family of NTFs is composed of GDNF, neurturin (NRTN), artemin (ARTN) and persephin (PSPN) (Airaksinen and Saarma, 2002). After binding to co-receptors of the GFRα family (GDNF to GFRα1, NRTN to GFRα2, ARTN to GFRα3, and PSPN to GFRα4), the complex consisting of GDNF family ligand (GFL) and GFRα can bind to and activate the transmembrane receptor tyrosine kinase RET. Activated and transphosphorylated RET uses similar signaling cascades as Trk (MEK/MAPK and PI3K/Akt pathways), resulting in neuronal differentiation, survival and growth. Furthermore, GDNF has also been found to signal through other alternative routes by interactions with the neural cell-adhesion molecules (NCAM) and syndecan-3 (Bespalov et al., 2011; Paratcha et al., 2003), affecting neuronal morphology, migration, and synapse formation.
Components of both BDNF and GDNF signaling machinery, i.e., expression of co-receptors and receptors, are present on dopamine neurons, and these cells are capable of retrograde transport of the NTFs (Mufson et al., 1994; Nosrat et al., 1997; Numan and Seroogy, 1999; Seroogy et al., 1994; Tomac et al., 1995). In the adult brain, GDNF and NRTN are best known for their actions on dopamine neurons (Airavaara et al., 2012; Domanskyi et al., 2015). Both GDNF and NRTN can protect and restore the nigrostriatal dopamine circuitry in animal models of PD, and they have been tested in human clinical trials for PD, with variable and mainly disappointing results (Rangasamy et al., 2010). In the intact rodent brain, GDNF boosts the dopaminergic function by increasing dopamine turnover, augmenting stimulus-induced dopamine release, as well as inducing sprouting of tyrosine hydroxylase-reactive fibers (Cass et al., 1999; Hebert et al., 1996; Hudson et al., 1995). Although the most intriguing effect of BDNF in the intact CNS may be its role in regulating long term potentiation (LTP) (Kang and Schuman, 1995; Lohof et al., 1993), infusion of BDNF into intact rat brain also increases dopamine turnover, induces contralateral rotations after amphetamine administration, and affects the firing rate and number of spontaneously active dopamine neurons (Altar et al., 1992; Shen et al., 1994). In addition, both BDNF and GDNF signaling promotes survival of embryonic midbrain dopamine neurons in vitro (Hyman et al., 1991; Lin et al., 1993).
Despite the strong evidence that BDNF and GDNF can affect the brain dopamine system, they do not seem to be crucial for the development of dopamine neurons in vivo. Full knockout of BDNF (Ernfors et al., 1994), GDNF, GFRα1 or RET is lethal as the mice die soon after birth (for review see Airaksinen and Saarma, 2002). However, virtually no defects in the dopamine system of these mice have been found. Studies done on conditional GDNF knockout mice report conflicting findings. In the first study, tamoxifen induction was used to remove GDNF from adult mice and reported a marked loss of dopamine neurons accompanied with progressive, but rather mild, changes in motor behavior (Pascual et al., 2008). However, using a different conditional GDNF knockout mouse model, Kopra et al. did not find any significant changes in the adult dopaminergic system (Kopra et al., 2015). The results from Kopra and colleagues are supported by findings from studies on conditional knockout of RET, where in two separate studies a decline in the mouse brain dopaminergic system was observed only in aged RET deficient mice (Jain et al., 2006; Kramer et al., 2007). Moreover, low levels of GDNF in heterozygous GDNF+/− mice have been associated with an earlier age-dependent drop in TH-reactive neurons in the substantia nigra (Boger et al., 2006). Although in vivo studies show that GDNF can act as a target-derived NTF for the developing dopamine neurons and prevent the natural loss of dopamine neurons that occurs shortly after birth, this does not result in alterations in dopamine cell numbers in the adult substantia nigra (Burke, 2006; Oo et al., 2003).
Altogether, surprisingly small changes have been observed in the CNS in both conventional and conditional NTF knockout mice. We know that a wide variety of NTFs are present in the CNS, and when a single NTF gene is knocked out, the expression of those that remain are modulated as an adaptation to the missing activity. In addition, exogenous NTF administration into the brain of adult animals can have dramatic effects, indicating that NTF signaling systems are still present and that endogenous NTFs have an impact on the adult CNS. However, the exact roles of NTFs during the development and especially the maintenance of the dopamine circuitry in the adult brain still remain unclear. Studies on conditional knockouts indicate that while mature dopamine neurons can stay fully functional in the absence of support from specific NTFs, NTF signaling may serve important functions in situations where the CNS is challenged. This could include defense against age-related build-up of injuries and deterioration of the CNS, as well as adaptation to changes in neuronal activity induced by, e.g., drugs of abuse. In addition, a change in NTF expression caused directly by a substance of abuse or indirectly by altered neuronal activity could mediate remodeling of neuronal function and synaptic plasticity. On the other hand, neuroadaptive changes brought on by abused drugs may be altered by (endogenous or exogenous) NTFs. Taking into account the possible importance of NTFs for both the neurobiology and treatment of CNS disorders, including addiction, it would be of great importance to clarify the role of NTFs in the maintenance and adaptation of specific neuronal populations in adult mammals.
Behavioral models to study drug rewards and craving
Because this special issue is intended for a broad audience, we summarize below the most commonly used behavioral models to study drug reward, relapse and craving. The defining features of addictive drugs in laboratory animals are that drugs of abuse can increase the time in drug-paired chamber in conditioned place preference, and in self-administration paradigms the animals are willing to respond and perform a task to earn a drug dose. Conditioned place preference is a classical Pavlovian conditioning procedure to study rewarding effects of abused drugs. In the training phase the drug is associated with the paired compartment and not with the other area. The training phase, when the animal receives the drug, is often repeated approximately four times, and after this, the time spent in each area is measured in the absence of drug. An increase in the time spent in the drug-paired compartment can be viewed as a measure of rewarding effects (Mucha et al., 1982). Drug self-administration is a procedure where laboratory animals perform a voluntary movement in order to obtain a drug. This movement can be a nose-poke or lever press. In the self-administration paradigm the drug itself functions as a positive reinforcer to increase frequency of the drug-delivery action (Weeks, 1962). Positive reinforcement in operant conditioning occurs if the presentation of the drug following a response increases the likelihood of the response. The reinstatement of drug use is a commonly used model for relapse where after a period of extinction reinstatement of behavior is usually triggered by one or a combination of three main factors: stress, environmental cues and re-exposure to the drug or drug-priming (Bossert et al., 2013; Shaham et al., 2003). Incubation of drug craving (Grimm et al., 2001; Lu et al., 2004b; Pickens et al., 2011) is a motivational process derived from the findings that there is time-dependent increase in cue-induced cocaine, amphetamine or heroin seeking after withdrawal from self-administration. In incubation of drug craving studies, the drug seeking behavior is tested by extinction tests after drug administration.
GDNF and BDNF in drug seeking behavior in laboratory animals
Ghitza et al. (2010) conclude in their review that GDNF or BDNF can either facilitate or inhibit drug-taking behaviors depending on the drug type, the brain site, the addiction phase and the time interval between BDNF/GDNF manipulations and reward- and relapse-related behavioral assessments (Ghitza et al., 2010). In studies on incubation of cocaine or heroin craving administration of BDNF or GDNF in the mesocorticolimbic system have been shown to increase craving (Airavaara et al., 2011a; Lu et al., 2004a; Lu et al., 2009). On the other hand, several alcohol studies have shown that GDNF has acute inhibitory effects on drug-seeking behavior (Carnicella et al., 2008; He and Ron, 2006). Similar effects have been observed with local acute BDNF injections. Thus, what appears to be an important variable for how NTFs modulate psychostimulant, heroin or morphine -induced behavior is the experimental settings; and in the case of alcohol GDNF and BDNF seems to have inhibitory effects on alcohol seeking. Moreover, in alcohol seeking, GDNF most likely plays a role in the VTA while the action of BDNF is localized within the dorsal striatum. In case of opiates and stimulants, the site specific actions between GDNF and BDNF on drug seeking behavior are quite similar. Overall, the effects of BDNF and GDNF on addiction-related behavior seem to depend on the drug of abuse and experimental settings. Recent results in the field are summarized below and in Table 1.
Table 1.
Effect of GDNF and BDNF on drug-seeking behavior in laboratory animals
| Drug | Drug administration | GDNF or BDNF | Brain area/Animal | Results | Reference |
|---|---|---|---|---|---|
| Alcohol | Single administration | GDNF endogenous | VTA/rat | Upregulation GDNF mRNA and protein levels | (Ahmadiantehrani et al., 2014) |
| Single administration | GDNF endogenous | NAc/rat | No alterations GDNF mRNA and protein levels | (Ahmadiantehrani et al., 2014) | |
| Intermittent access 20% ethanol two-bottle choice | GDNF knockdown | VTA/rat | Increased ethanol consumption | (Ahmadiantehrani et al., 2014) | |
| Transition from moderate to excessive 20% ethanol drinking | GDNF knockdown | NAc/rat | Increased ethanol consumption | (Barak et al., 2015) | |
| Moderate and chronic ethanol consumption, free bottle choice | BDNF endogenous | Hippocampus/mouse | Increased BDNF expression | (Stragier et al., 2015) | |
| Operant self-administration of 10% ethanol | BDNF endogenous | DLS/rat | Increased BDNF expression | (Jeanblanc et a., 2013) | |
| Morphine | Subcutaneous morphine injection | GDNF endogenous | VTA/rat | (Li et al., 2014) | |
| Subcutaneous or intermittent intraperitoneal morphine injection | BDNF endogenous | VTA/mouse | Decreased BDNF expression | (Koo et al., 2012) | |
| CPP | BDNF knockdown | VTA/mouse | Enhanced drug rewarding effect | (Koo et al., 2012) | |
| CPP | BDNF infusion | NAc/mice | No effect on drug rewarding | (Koo et al., 2012) | |
| Chronic intraperitoneal morphine injection | BDNF endogenous | VTA/rat | Suppressed BDNF expression | (Koo et al., 2015) | |
| Chronic intraperitoneal morphine injection | BDNF endogenous | VTA/mouse | Suppressed BDNF expression | (Koo et al., 2015) | |
| Morphine withdrawal | BDNF endogenous | Hippocampus, frontal cortex, midbrain/rat | Increased BDNF expression | (Peregud et al., 2015) | |
| Methamphetamine | Amphetamine i.p. injection | BDNF receptor TrkB | Caudate putamen, NAc/rat | Activation TrkB/BDN F signaling | (McGinty et a., 2011) |
| Chronic methamphetmaine i.p. injection | BDNF alteration | BDNF HET mice | No locomotor sensitization | (Manning et al., 2015) | |
| Self-administration | BDNF endogenous | Hippocampus/rat | Increased BDNF expression | (Galinato et al., 2015) | |
| Incubation of methamphetamine craving model | BDNF mRNA and TrkB mRNA endogenous | Dorsal striatum/rat | Increased BDNF mRNA and TrkB mRNA expression | (Li et al., 2015) |
Alcohol
GDNF
Ahmadiantehrani et al. (2014) observed an upregulation in endogenous levels of GDNF mRNA and protein in the rat VTA 10 hours after a single administration of 20% ethanol. Interestingly, no alterations in GDNF mRNA were found in the NAc, indicating that the ethanol-mediated effects on GDNF expression are restricted to the VTA (Ahmadiantehrani et al., 2014). Additionally, knockdown of GDNF expression in the VTA of ethanol-naive rats led to significantly higher levels of ethanol consumption compared to the control group. These data suggest that endogenous GDNF in the VTA suppresses a rapid increase in ethanol consumption during the first week of ethanol drinking. A downregulation of GDNF in the rat NAc also facilitates the transition from moderate to excessive drinking of 20% alcohol. Particularly, knockdown of GDNF expression in the NAc results in a very acute relapse after abstinence, whereas overexpression of GDNF in the mesolimbic system removes the escalation to excessive alcohol consumption (Barak et al., 2015).
In light of previous findings
Previous studies have shown a rapid reduction in operant self-administration of alcohol after single administration of GDNF into the rat VTA (Carnicella et al., 2008; He et al., 2005) and particularly the reduction was in lever presses for 20% ethanol after the GDNF infusion (Carnicella et al., 2008). Together with the more recent findings described above the data suggest that GDNF is a potent inhibitor of excessive ethanol consumption, most likely via its actions in the VTA.
BDNF
Moderate, chronic ethanol consumption has been observed to induce epigenetic changes in the hippocampus of mice (Stragier et al., 2015). Particularly, epigenetic modifications to the Bdnf gene correlate with increased BDNF expression and subsequent stimulation of neurogenesis in the subgranular zone of the dentate gyrus through TrkB receptor activation. Moderate consumption of 10% ethanol also increases BDNF expression within the rat dorsolateral striatum, and direct infusion of BDNF into the dorsolateral striatum decreases operant self-administration of a 10% ethanol solution (Jeanblanc et al., 2013). In addition, the BDNF-mediated reduction in ethanol consumption was blocked by inhibition of MAPK activity, and also by the protein synthesis inhibitor cycloheximide.
In light of previous findings
Taken together, recent results support earlier studies on the role of BDNF in alcohol consumption (Jeanblanc et al., 2006; Logrip et al., 2008; Logrip et al., 2009) suggesting that BDNF is one of the key factors in the dorsal striatum that regulate ethanol intake.
Morphine
GDNF
GDNF mediates negative regulatory effects on chronic morphine-induced neuroadaptations, and NCAM signaling seems to be involved in this process (Li et al., 2014). NCAM is widely expressed in the rat VTA, including all dopamine neurons. Although NCAM expression levels remain unchanged, the phosphorylation of NCAM-associated focal adhesion kinase is increased by GDNF, and this upregulation can be inhibited by pre-treatment with an antibody that blocks NCAM function. Pre-treatment with the antibody antagonizes the inhibiting effect of GDNF on neuroadaptations induced by chronic morphine exposure, including a decrease in the number and length of neurites, decreased size of cell bodies and an increase of tyrosine hydroxylase in the VTA dopamine neurons (Li et al., 2014).
In light of previous findings
When comparing more recent results described above to the previous results with morphine the overall picture is not clear. In one study heterozygous GDNF+/− mice displayed enhanced conditioned place preference (Niwa et al., 2007) while others have reported the conditioned place preference to be similar in GDNF+/− and wild-type mice, but of shorter duration in GDNF+/− mice (Airavaara et al., 2007). In studies of heroin craving GDNF injections in the NAc, but not VTA, increase the extinction response after withdrawal (Airavaara et al., 2011a). Similar to the study by Li et al. (2014) GDNF has been also earlier found to block morphine-induced tyrosine hydroxylase upregulation (Messer et al., 2000). Additional studies are needed to better establish the role of GDNF in morphine addiction.
BDNF
BDNF has been identified as a negative modulator of morphine action (Koo et al., 2012). Chronic morphine, given to mice by subcutaneous pellets or intermittent intraperitoneal injections, suppresses BDNF expression in the mouse VTA, and this decrease in BDNF can promote rewarding and locomotor responses to morphine by increasing dopamine neuron excitability. Accordingly, BDNF infusion into the VTA suppresses morphine–induced conditioned place preference, whereas an optical stimulation of dopamine terminals in the NAc is able to completely reverse the suppressive effect of BDNF on morphine reward.
Quantitative chromatin immunoprecipitation with antibodies recognizing differentially phosphorylated forms of RNA polymerase II together with a comprehensive analysis of epigenetic regulation of Bdnf gene suggested that exposure to chronic morphine can increase the stalling of RNA polymerase II at Bdnf promoters in the rat VTA (Koo et al., 2015). Furthermore, morphine seems to suppress the binding of phospho-CREB (cAMP response element-binding protein) and NURR1 (nuclear receptor related-1) to Bdnf promoters in the VTA, resulting in decreased expression of BDNF. In line with this, overexpression of NURR1 in the VTA was shown to decrease morphine-induced place preference, whereas a local knockout of Bdnf could abolish this effect (Koo et al., 2015).
Nitric oxide (NO) signaling can also regulate the expression of BDNF, GDNF and their receptors in different brain regions of morphine-dependent rats after spontaneous morphine withdrawal (Peregud et al., 2015). In these experiments morphine withdrawal was accompanied by an increased expression of BDNF mRNA in the frontal cortex, hippocampus, and midbrain, while the mRNA levels of the BDNF receptor TrkB were increased only in the frontal cortex. Interestingly, the authors showed that administration of the NO synthase inhibitor L-NG-nitroarginine methyl ester (L-NAME) during morphine intoxication prevented the increase in BDNF and TrkB mRNA expression, suggesting that NO signaling during the development of morphine dependence can modulate the BDNF/TrkB pathway (Peregud et al., 2015).
In light of previous findings
Previous studies have shown that repeated heroin exposure increases BDNF levels in the VTA and that intra-VTA infusion of BDNF causes a shift from dopamine-dependent to dopamine-independent opiate reward system (Vargas-Perez et al., 2009). However, as described above, chronic morphine decreases BDNF expression in the VTA (Koo et al., 2012) which may be caused by observed changes in transcription activity. Moreover, the observed increase of BDNF mRNA in hippocampus after morphine withdrawal, in general, complements an earlier study that found an increased expression of BDNF mRNA in hippocampus of mice subjected to morphine (Wan et al., 2011). Taken together, with respect to existing studies on effects of morphine and other opioids in relation to BDNF functioning, the relationship remains unclear.
Methamphetamine
BDNF
Recently Galinato et al. (2015) reported that self-administration of methamphetamine increases BDNF expression in the rat hippocampus. Furthermore, enhanced level of BDNF did not cause alteration of total TrkB or phosphorylated TrkB expression 16–20 h after the last self-administration of the drug (Galinato et al., 2015). In addition, McGinty et al. (2011) observed an increase of phosphorylated TrkB level, but not total TrkB, in the caudate putamen and NAc and not in the dorsomedial PFC, two hours after a single i.p. injection of amphetamine in rats, suggesting that BDNF/TrkB activation might occur in a time-dependent manner. Interestingly, significant increase in mRNA expression of BDNF and TrkB was observed in the Fos-positive neurons of rat dorsal striatum in incubation of methamphetamine craving (after prolonged withdrawal) model (Li et al., 2015). In addition, rats that received an infusion of the tyrosine kinase inhibitor K252 into the dorsal striatum 20 min prior to amphetamine administration showed an increase in vertical activity (McGinty et al., 2011). These results are consistent with other studies, where stimulation of TrkB signaling has been shown to attenuate methamphetamine-induced hyperlocomotion (Ren et al., 2014), whereas heterozygous BDNF+/− mice display impaired locomotor sensitization to acute amphetamine administration (Manning et al., 2015). Conflicting profiles of behavioral changes in wild-type and heterozygous BDNF+/− mice have also been demonstrated after chronic methamphetamine treatment.
In light of previous findings
Over last five years, the studies on the role of BDNF in methamphetamine-induced behavior have extended our knowledge and provided new insights. It was previously shown that there are increased total TrkB levels in the hippocampus and the NAc shell after amphetamine-induced conditioned place preference (Shen et al., 2006), but these data were not confirmed in later studies by McGinty et al. (2011) and Galinato et al. (2015). The more recent data shows changes in phosphorylated form of TrkB. Thus, BDNF/TrkB signaling plays a role in methamphetamine-induced changes to behavior in a time-dependent manner.
MicroRNAs and natural antisense transcripts related to NTFs
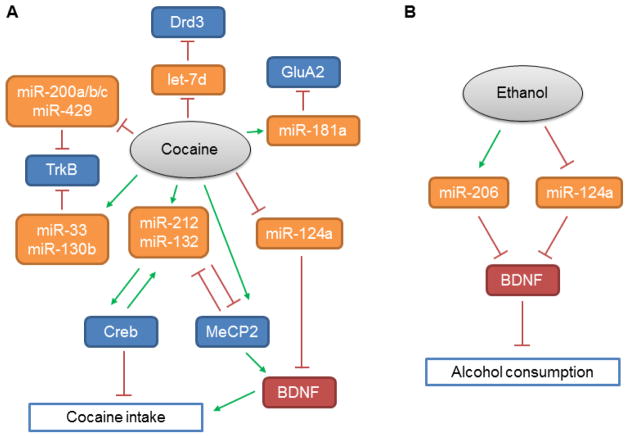
MicroRNAs play an essential role in regulating specificity of BDNF-induced protein synthesis (Huang et al., 2012). Multiple microRNAs target BDNF (Varendi et al., 2015) and GDNF (Kumar et al., 2015), therefore, drug-induced changes in expression of such microRNAs may regulate addiction-related behaviors via affecting BDNF and/or GDNF levels. MicroRNAs are ≈22 nucleotides-long RNA molecules that destabilize mRNA and/or suppress protein translation via binding to complementary sites in the 3’ untranslated region (UTR) of target mRNA. MicroRNAs are predicted to control the activity of about half of all protein coding genes (Krol et al., 2010). Each microRNA has a potential to regulate hundreds of transcripts, mediate a formation of large-scale regulatory networks, and enable a crosstalk between different cellular pathways (Salmena et al., 2011; Sumazin et al., 2011). MicroRNAs are processed from precursor molecules by two double-strand RNA-specific nucleases, Drosha and Dicer (Krol et al., 2010). A genetic ablation of Dicer in the mouse results in the disruption of microRNA processing pathway, leading to the loss of mature forms of microRNAs (Konopka et al., 2010; Vinnikov et al., 2014). In particular, the loss of Dicer in embryonic dopamine neurons severely impairs the development of the dopaminergic system (Kim et al., 2007). Through their ability to affect the translation of multiple synaptic proteins and regulate local translation of several mRNAs in the dendrites, microRNAs serve as important modulators of synaptic plasticity (Heyer and Kenny, 2015; Im and Kenny, 2012; Schratt, 2009). Indeed, the disruption of microRNA biogenesis pathway in hippocampal neurons affects learning and memory (Konopka et al., 2010). Since addiction can also be viewed as maladaptive learning (Mameli and Luscher, 2011), the studies on microRNA functions in regulating addiction-related behaviors and drug-induced plasticity in mesolimbic dopamine neurons are attracting considerable interest (Dreyer, 2010; Eipper-Mains et al., 2012; Heyer and Kenny, 2015; Im and Kenny, 2012). Several microRNAs have been implicated in drug addiction, including miR-132 and miR-212 that modulate CREB and BDNF signaling in the striatum and control cocaine intake under extended access conditions (Hollander et al., 2010; Im et al., 2010). Chronic cocaine administration affects the expression levels of miR-124a, let-7d and miR-181a that, in turn, regulate several plasticity-related mRNAs (including BDNF that is a putative target of miR-124a) in the mesolimbic dopamine system (Chandrasekar and Dreyer, 2009). Sequence analysis of microRNAs in purified striatal post-synaptic densities in mice after chronic cocaine exposure identified a set of microRNAs predicted to target TrkB (Eipper-Mains et al., 2011) (Figure 1A). Downregulation of miR-124a and upregulation of BDNF has also been observed following ethanol intake (Bahi and Dreyer, 2013). Similarly, upregulation of miR-206 in the medial PFC contributes to escalated alcohol consumption, possibly, via downregulation of BDNF (Tapocik et al., 2014) (Figure 1B).
Figure 1.
MicroRNAs in drug addiction. Selected microRNAs affected by (A) cocaine administration or (B) ethanol drinking regulate BDNF signalling and related pathways promoting or inhibiting drug uptake.
Occurrence of natural antisense transcripts in BDNF (Pruunsild et al., 2007) and GDNF (Airavaara et al., 2011b) loci adds yet another level of complexity to the regulation of these NTFs. It has been shown that the knockdown of BDNF antisense (BDNF-AS) or GDNF antisense (GDNF-AS) transcripts leads to upregulation of BDNF or GDNF mRNA levels, respectively, possibly through modification of repressive chromatin marks (Modarresi et al., 2012). These natural antisense transcripts have not been studied in the addiction studies yet. Interestingly, some natural antisense transcripts may also compete with microRNA(s) for binding to the sense mRNA leading to its increased stability (Faghihi et al., 2010). Recent genomic analyses have revealed a large population of long noncoding RNAs (lncRNAs) among many thousands of noncoding RNAs (Jia et al., 2010). Moreover, it has been shown that expression of lncRNAs is high in the brain, however, whether they play role in addiction is not clear. It remains to be investigated whether exposure to drugs of abuse will have an effect on natural antisense transcripts-mediated regulation of BDNF and GDNF levels. Given the substantial role of BDNF and GDNF in drug addiction, studies of antisense genes in drug-seeking behaviors by gene-manipulation in animals would be of great importance.
Future perspectives for the studies exploring neurobiology of GDNF and BDNF in addiction-related behaviors
The studies presented above, together with results from earlier work (reviewed by (Bolanos et al., 2005; Carnicella and Ron, 2009; Ghitza et al., 2010; Niwa et al., 2007; Pierce and Bari, 2001; Ron and Janak, 2005; Russo et al., 2009), strongly indicate the involvement of GDNF and BDNF in processes underlying neuroadaptation and establishment of addiction-related behavior induced by drugs of abuse. However, we are still far from understanding the exact mechanisms of interactions between NTFs and drugs of abuse, and how these interactions can create or modify behavioral patterns typical to drug abuse. In order to try to increase our knowledge we can take advantage of technologies that have evolved during the last decade. Below we summarize the future perspectives that in our opinion will advance the NTF field. These technologies include genetic tools that allowed to create transgenic rats, which eventually enable us to take full advantage of gene manipulation in studies of complex addiction-related behaviors. Since the available tools for modulation of gene expression in mice are still much more versatile, new studies in mice could include the studies of addiction-related behavior in a social context.
Transgenic rats as a tool to study addiction-related behavior
Animal studies involving NTFs gene modifications are all done in mice, whereas all the rat studies include either ectopic overexpression with viral vectors or injections of recombinant proteins, and both of these approaches often lead to unnaturally high concentration of NTFs in the brain. The studies with activity blocking antibodies also include many methodological limitations. Studies with viral vectors expressing shRNAs often lead to only partial blocking of endogenous NTF activity. Over the past decade, advancements in transgenic technology have greatly aided in the development of transgenic rats. Since the 80’s the mouse has been the predominant mammalian species to undergo transgenesis to create models of human disease and understand gene function in normal and pathological processes. It was not until 2008 when a new method to establish mouse ESCs was discovered and shown to be applicable to rats (Ying et al., 2008) and rat ES cells were established (Buehr et al., 2008; Li et al., 2008). The ability to culture rat spermatogonial stem cells (Hamra et al., 2005) and use them to generate transgenic rats (Izsvak et al., 2010) has also expanded the availability of transgenic rats.
Given the prevalence of transgenic mice for studying the nervous system, why transgenic rats for addiction? Since the early part of the 20th century, rats have been the preferred rodent model for studying the neurobiology of cognition and behavior. For example, the rats are preferred for modeling aspects of drug abuse-related behaviors such as impulsivity, self-administration, and incentive salience (Parker et al., 2014). The gradual decline in use of rats for studies in neurobiology follows the gradual increase in transgenic mouse models, the availability of the full sequence of the mouse genome several years before the rat and per diem charges being greater for rats compared to mice. There has also been a lag in the molecular tools essential for creating and characterizing the rats such as complete verified annotation of the rat genome and antibodies specific to rat antigens, respectively. However, the ability to successfully manipulate rat stem cells using CRISPR technology (Chapman et al., 2015; Yamamoto et al., 2015) and the emergence of new transgenic rat resources will continue to expand the transgenic rat models needed to understand the molecular contributions of NTFs in addiction and other neurological diseases. At the moment there are neither NTF knockout nor conditional knockout rats available, nor there are any rat lines overexpressing NTFs in the brain. Many non-profit organizations are now developing and distributing transgenic rats. For example, the National Institute on Drug Abuse, NIH (United States) is currently developing novel transgenic rats for studies of addiction and other neurological diseases (http://irp.drugabuse.gov/OTTC/rats.php). The Rat Resource and Research Center (http://www.rrrc.us) in the United States is a non-profit organization that serves as a repository for transgenic rats. Lastly, the National BioResesource project for the rat in Japan (http://www.anim.med.kyoto-u.ac.jp/NBR/) also serves as a repository for transgenic rats. As future studies of addiction and the role of NTFs progress, development of rat models of addiction should be considered.
CRISPR-Cas9 for controlling gene expression
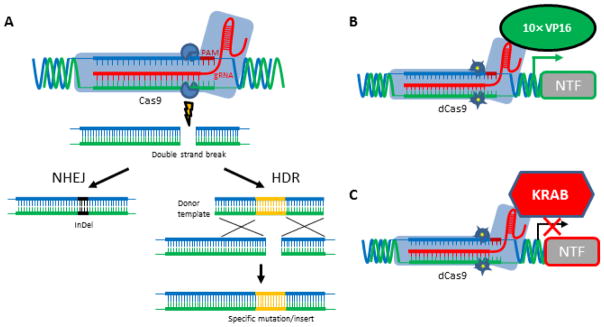
Recent advances in RNA-directed (aka programmable) DNA endonucleases, namely the adaptation of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) system for use in eukaryotic cells, have drastically reduced the difficulty, time, and expense usually associated with genome engineering (Cong et al., 2013; Jinek et al., 2012). This new genome editing technology provides an efficient tool for studies of NTF addiction biology. Now knock-out animal models can be produced simply by injecting an embryo with the programmable endonuclease Cas9 and a short synthetic RNA molecule called a guide RNA (gRNA) which is homologous to the target gene (Wang et al., 2013). Once inside the nucleus, the Cas9 protein/gRNA complex scans the genome for sequences homologous to the guide RNA. Once a match has been found, Cas9 produces a double-strand break. In mammalian cells, this type of lesion is typically repaired by non-homologous end joining (NHEJ), an error-prone process that produces small insertions or deletions (InDels) (Figure 2A). When an InDel occurs within the coding region of a gene, it has a 2/3 chance to produce a frameshift which should lead to premature termination of the gene’s open reading frame. Mutations created in this fashion can be isolated and phenotypically characterized, and/or used to found a breeding colony, as long as the targeted gene is not essential for viability and reproduction.
Figure 2.
CRISPR-Cas9 for controlling gene expression. (A) Binding of 20 nucleotides (nt) within the guide RNA (gRNA) to the complementary genomic DNA sequence preceding the 3 nt protospacer-associated motif (PAM) targets Cas9 to induce a site-specific double-strand break that is repaired by non-homologous end joining (NHEJ) or homology-directed repair (HDR). NHEJ frequently produces small insertions or deletions (InDels) that can lead to frameshift mutations in the target gene. In the presence of donor DNA template HDR leads to the insertion (knock-in) of specific DNA sequence to the target locus. (B) Activation or (C) inhibition of target gene transcription can be achieved by fusing the “dead” Cas9 (dCas9) protein carrying two point mutations blocking nuclease activity of Cas9 to VP16 transcriptional activator domains (10× VP16) or to a repressive chromatin modifier domain (KRAB), respectively. Images modified from (Charpentier and Doudna, 2013; Gilbert et al., 2014).
Specific alleles (i.e. amino acid substitutions or loxP insertions) as well as larger targeted knock-in transgenes can also be generated with this technique, but require the addition of a donor template when preparing the injection cocktail. A donor template is a double or single stranded DNA fragment that codes for the intended modifications flanked by arms that are homologous to the sequences that lie on either side of the Cas9-mediated breakpoint. The presence of these flanking sequences in the donor shifts the cellular repair processes from NHEJ to a homology-directed repair (HDR) mechanism, which effectively transfers the new information contained in the donor template to the genome at the site of the break (Figure 2A). This technique has been used to produce specific point mutations, as well as insertion of loxP sites and epitope tags (Ran et al., 2013; Shao et al., 2014; Wang et al., 2013; Yang et al., 2013).
CRISPR-based genomic modification can also be performed on post-natal animals using Cre-dependent transgenes and/or viral vectors to deliver the Cas9 and gRNA components. Combinations of adeno-associated viral (AAV) vectors have been used to convey all three CRISPR components (Cas9, gRNAs, and donor template) (Platt et al., 2014; Swiech et al., 2015). This also provides an important tool for studies of NTFs in addiction biology, because NTF level and levels of several NTFs at the same time can be manipulated, and this can be done before, during or after self-administration. Additionally, a tissue-specific deletion can be achieved when the Cas9 protein is provided not via the viral vector, but instead supplied by the host cell as a Cre-dependent transgene (Akagi et al., 1997). When combined with a tissue-specific Cre driver transgene, Cas9 is expressed only in cells that also express Cre recombinase.
Due to limitations imposed by transduction efficiency and the independent repair of each break in each transduced cell, this method produces a genotypically diverse population within the affected region. Therefore, the phenotype presented by this system may not be completely identical to a germline-derived knock-out of the same gene. While viral-mediated CRISPR may not be as penetrant as a traditional knockout derived from an embryonic stem cell, it has certain advantages such as no added expenses associated with creating/maintaining a transgenic colony, application of the CRISPR-mediated gene deletion to the adult animals to avoid possible developmental phenotype, and the ability to multiplex target genes by combining different gRNA vectors.
Another method for using CRISPR to manipulate target gene expression has been developed which bypasses the making of mutations all together. Here, the nuclease activity of Cas9 has been knocked out by two point mutations, while leaving the RNA-directed DNA binding activity unaffected. By fusing the dead Cas9 (dCas9) to the transcriptional activator VP16, and combining it with gRNAs homologous to your target gene’s promoter region, one is able to generally increase the transcription of the target gene’s mRNA (Cheng et al., 2013) (Figure 2B). With this method more physiological levels of NTFs expression can be achieved in desired tissues. However, this method can cause the overexpression also in cells that do not normally express NTFs. One way to limit the overexpression to cells that expresses the NTF gene natively, is the genetic ablation of the 3’UTR as demonstrated for Gdnf (Kumar et al., 2015). Conversely, by fusing dCas9 to a repressive chromatin modifier domain (KRAB), one can effect gRNA-dependent gene silencing (Gilbert et al., 2014) (Figure 2C). Overall, the use of CRISPR-based technology can allow the development of novel transgenic animals and viral vector-mediated transgenesis to further understand the role of NTFs in addiction and altered NTF levels can be used in studying drug self-administration, reward, relapse or craving.
Behavioral studies in social context in mice and rats
Most addiction-related behavioral studies where voluntary consumption of drugs of abuse is associated with cues have been carried out in environments where animals are single housed. This is particularly true for studies where the role of neurotrophic factors BDNF or GDNF is related to the effects of drugs of abuse in combination with cue-induced behavioral effects (Ghitza et al., 2010). Environmental enrichment has been associated with increased levels of BDNF and CREB activity in the NAc (Green et al., 2010). Since endogenous NTFs are regulated by the environment (Ickes et al., 2000; Rossi et al., 2006), it would be important to study whether the environmental and social effects play a role in the addiction-related behavior. Although rat models are well validated and indispensable tools in the addiction field, mouse studies have revealed several issues that should be considered. Below we discuss why research in addiction field, and particularly from the viewpoint of NTFs, should consider the social context.
Mice have become the subjects of choice for basic biomedical research, especially for animal models addressing the role and function of genes in physiology and pathology (Collins et al., 2007; Rosenthal and Brown, 2007). Ambitious projects have been launched with the aim of identifying the functions of every single gene (Austin et al., 2004; Auwerx et al., 2004). These new mouse lines serve as invaluable populations for refined research in various fields of biomedicine. However, the short history of behavioral phenotyping of genetically modified mice has been confronted with number of challenges (Sousa et al., 2006), and meaningful analysis can be achieved by comprehensive, systemic and systematic phenotyping (Beckers et al., 2009). The problems associated with the genetic background of mutant mice were recognized quite early (Gerlai, 1996; Lipp and Wolfer, 1998) and several recommendations for controlling the background are available (Lipp and Wolfer, 2003; Silva et al., 1997; Wolfer et al., 2002). Behavioral testing per se has posed several caveats to research community. Initially, the concept of behavioral test batteries was accepted – comprehensive testing of various domains was thought to minimize the occurrence of false positive and negative findings (Crawley and Paylor, 1997). However, soon it was realized, that application of several tests on the same individual could also affect the results – naïve mice appeared to behave sometimes differently than experienced mice (McIlwain et al., 2001; Voikar et al., 2004). Moreover, the studies reporting discrepancies between the laboratories testing the same strains or mutants are not rare (Crabbe et al., 1999; Crestani et al., 2000). The experimenter and handling of the mice have been shown to be the major sources of variability (Chesler et al., 2002; Crabbe et al., 1999). In addition, single-housing is often used for various reasons during experiments. However, such isolation is a well-known stressor for social species (Valzelli, 1973; Voikar et al., 2005). Therefore, automated tests with minimal human interference in species-specific social environment could be of high priority in the future (Spruijt and DeVisser, 2006; Tecott and Nestler, 2004).
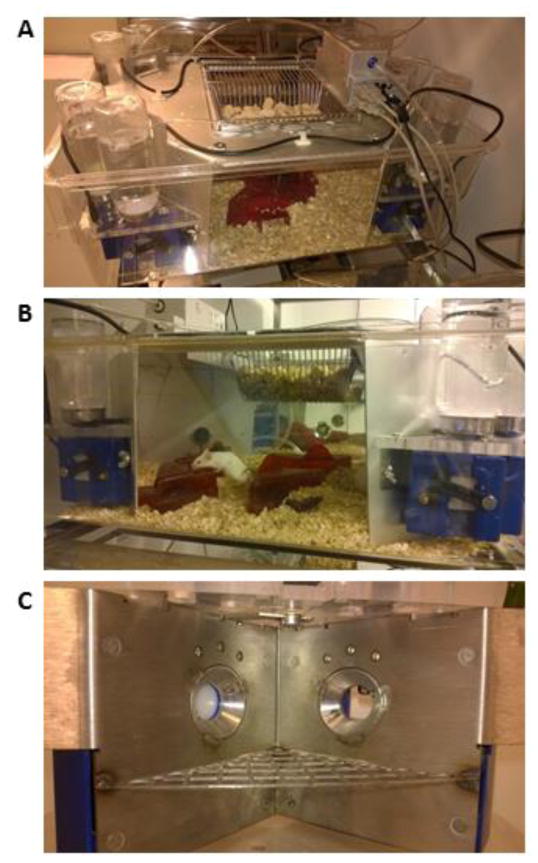
Animal models in addiction research and studies on the role of NTFs are influenced by the same challenges as listed above – increased use of genetically modified mice in the background of extensive history of research carried out in rats. However, transfer of rat paradigms is not a straightforward task. Therefore, development and validation of reliable tests for studying addictive behavior in mice is important. Number of applications (at least 10) has been developed during the last decade for testing mice in their home cage environment (reviewed in Spruijt and DeVisser, 2006; Richardson 2015). These methods can contain specific experimental modules, are fully automated and, importantly, the handling of animals by humans is limited to routine cage cleaning. However, most of the available systems still require single housing. In contrast, one of these applications, the IntelliCage (Figure 3, A–B) allows monitoring of individual animals in social setting. Parallel studies carried out with IntelliCage in different laboratories have shown to produce consistent behavioral phenotypes for inbred strains (Codita et al., 2010; Endo et al., 2011; Voikar et al., 2010), suggesting that this approach may, indeed, provide standardized and reproducible way to use this kind of social concept in experiments along with higher ethological validity. The main application of the IntelliCage so far has been in monitoring the exploratory activity and cognitive functions of inbred and mutant mouse strains (Vannoni et al., 2014). The number of publications where IntelliCage has been applied as a tool for behavioral testing is growing, although we have not found references directly addressing the role of NTFs in behavior. However, the method has been valuable for investigating the links between neurogenesis and behavior (Vannoni et al., 2014), in characterization of mouse models for Huntington’s disease (Knapska et al., 2013; Knapska et al., 2006; Kobayashi et al., 2013), Alzheimer’s disease (Codita et al., 2010; Ryan et al., 2013; Weyer et al., 2011), Down syndrome (Faizi et al., 2011), autism (Puscian et al., 2014). In addition, IntelliCage has been used as either enriched or stressful environment and combined with measurement of changes in the levels of BDNF and glucocorticoids (Alboni et al., 2015; Branchi et al., 2013; Kulesskaya et al., 2014) provides an excellent ethological surrounding for modeling stress and depression-like behavior in mice. Overall, considering the role of NTFs in neuropsychiatric and neurodegenerative conditions on one hand, and flexible task design and longitudinal monitoring in animal home-cage on the other hand, we argue that these systems will be invaluable for future research (Alexandrov et al., 2015; Gomez-Marin et al., 2014; Richardson, 2015).
Figure 3.
The Intellicage is an apparatus designed to fit inside a large standard cage of 20 cm high, 55 cm long and 38 cm wide at the base. A–B) Top and side view of the IntelliCage; C) Inside view of the conditioning corner. The apparatus itself provides four recording chambers that fit into the corners of the housing cage. Access into the chambers (inner dimensions 75×75×105 mm) is provided via a tubular antenna (50 mm outer and 30 mm inner diameter) reading the transponder codes. The chamber, equipped with a proximity sensor, contains two openings of 13 mm diameter (one on the left, one on the right) which give access to water-bottle nipples. These openings are crossed by photobeams recording nosepokes of the mice and the holes can be closed by small motorized doors, thus barring access to either or both water bottles in each corner. Above the openings, 3 LED’s are mounted for using as stimulus lights. In addition, air tube can be connected to the top of chamber for providing aversive airpuffs. Four triangular shelters are placed in the middle of the IntelliCage and can be used as sleeping quarters and as a stand to reach the food. Food is also scattered in the bedding. The IntelliCage is controlled by a computer with dedicated software, executing preprogrammed experimental schedules and registering visits to corner chambers, nosepokes to the door areas and licks at the nipple as parameters of mouse behavior.
Free-choice oral consumption of ethanol has frequently been used to study the genetic and neurobiological mechanisms underlying the alcohol drinking behavior in mice and rats (Yoneyama et al., 2008). Additionally, various operant or Pavlovian conditioning tasks have been applied for studying the rewarding properties of the ethanol – operant self-administration, conditioned place preference and conditioned taste aversion. It has been argued that home cage drinking is genetically correlated with conditioning assays (Green and Grahame, 2008). The IntelliCage corner (Figure 3, C) can be characterized as a small conditioning chamber allowing for successful and efficient testing of motivation, attention and impulsivity, reward and punishment (Knapska et al., 2013; Knapska et al., 2006; Kobayashi et al., 2013). In addition, it can be used as a system for measuring the taste function (Patrikainen et al., 2014), as in principle it is possible to have eight different concentrations or taste modalities in one cage. Based on our previous studies with GDNF and addiction-related behavior (Airavaara et al., 2011a; Airavaara et al., 2004; Airavaara et al., 2007), we are developing behavioral model in the IntelliCage for alcohol craving after withdrawal with focus on NTFs (Veremieva et al., 2015). The advantage of testing in home cage is that it is less stressful for the animals and less sensitive to environmental effects. For instance, the ethanol drinking scores in different laboratories have been closely comparable in the studies that otherwise revealed several robust effects of testing site (Crabbe et al., 1999). Therefore, the home-cage systems would have a great potential for improving the consistency and throughput in this type of studies by avoiding limitations associated with testing isolated animals. Only few recent studies have applied the IntelliCage for assessment of alcohol drinking (Mijakowska et al., 2015; Parkitna et al., 2013; Radwanska and Kaczmarek, 2012; Smutek et al., 2014). These studies have evaluated different addiction-like behaviors, including the motivation for drinking in progressive-ratio schedule reinforcement, persistent and compulsive alcohol seeking and taking during periods when alcohol is not available or is adulterated or paired with punishment and the intensity of relapse after alcohol withdrawal. In addition, an approach for mapping social interactions among the mice has been developed based on the sequence of entries into the cage corners.
In summary, many of the addiction-related behavioral studies are being conducted in isolated animals. For the future studies, it would be useful to compare the effects of addiction-related behaviors in social context versus isolation from perspective of NTFs biology. Comprehensive behavioral characterization of disease models requires novel approaches in basic research. One way to enhance understanding in mechanisms of pathology, progression of symptoms and effect of treatment is to apply automated monitoring combined with specific behavioral tests in familiar environment – animal home cage.
Conclusions
Drugs of abuse induce neuroadaptative processes in the brain, and these changes involve the regulation of BDNF and GDNF activity. Even though the exact role of GDNF and BDNF still is unclear, studies in the field clearly show the involvement of these NTFs in the development of addiction and in drug-seeking behavior. The link between drugs of abuse and NTFs appears to be a mutual process: abused drugs can affect the expression of NTFs, and NTFs can affect the outcome of drugs and modulate the drug-induced behavior. How GDNF or BDNF affect the drug-seeking behavior depends on the drug type, addiction phase, and the timing of GDNF/BDNF treatment in relation to drug administration. Furthermore, microRNAs and anti-sense transcripts have shown to regulate NTFs expression and addiction related behavior providing insight into the complexity of NTFs addiction biology. New technologies, such as assessing addiction-related behavior in a social context using IntelliCages, CRISPR/Cas9 genome editing, and development of transgenic rats provide unprecedented opportunities to further study the neurobiology of addiction and how addiction can be modified by BDNF and GDNF. In addition to these novel tools, there has been an impressive development of experimental models, such as tests used to evaluate reinstatement and incubation of drug craving. In many of these tests there is a major component of motivation, learning and memory underlying the behavioral changes that can be boosted or dampened by pharmacological manipulations. Studies on motivation, learning, and memory are extremely important far beyond their relevance to addiction diseases. Besides cognitive decline, major decreases in motivation are frequently observed in many neurodegenerative diseases including PD and AD, and the results from the addiction field can have tremendous implications for these diseases as well. It should be noted that motivation-related behavior is poorly studied in animal models of neurodegenerative diseases, and many behavioral tools could be adopted from the addiction field to further our understanding.
Acknowledgments
For funding MA, AD acknowledge Academy of Finland, MK Finnish alcohol research foundation.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadiantehrani S, et al. GDNF is a novel ethanol-responsive gene in the VTA: implications for the development and persistence of excessive drinking. Addict Biol. 2014;19:623–33. doi: 10.1111/adb.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Airavaara M, et al. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addict Biol. 2011a;16:261–72. doi: 10.1111/j.1369-1600.2010.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airavaara M, et al. Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci. 2004;20:2336–44. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- Airavaara M, et al. Identification of novel GDNF isoforms and cis-antisense GDNFOS gene and their regulation in human middle temporal gyrus of Alzheimer disease. J Biol Chem. 2011b;286:45093–102. doi: 10.1074/jbc.M111.310250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airavaara M, et al. Effects of repeated morphine on locomotion, place preference and dopamine in heterozygous glial cell line-derived neurotrophic factor knockout mice. Genes Brain Behav. 2007;6:287–98. doi: 10.1111/j.1601-183X.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Airavaara M, et al. Neurorestoration. Parkinsonism Relat Disord. 2012;18(Suppl 1):S143–6. doi: 10.1016/S1353-8020(11)70045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi K, et al. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 1997;25:1766–73. doi: 10.1093/nar/25.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni S, et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.191. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov V, et al. Reprint of: Highthroughtput analysis of behavior for drug discovery. Eur J Pharmacol. 2015;753:127–34. doi: 10.1016/j.ejphar.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Altar CA, et al. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89:11347–51. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CP, et al. The knockout mouse project. Nat Genet. 2004;36:921–924. doi: 10.1038/ng0904-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auwerx J, et al. The European dimension for the mouse genome mutagenesis program. Nat Genet. 2004;36:925–927. doi: 10.1038/ng0904-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci. 2013;38:2328–37. doi: 10.1111/ejn.12228. [DOI] [PubMed] [Google Scholar]
- Barak S, et al. Glial cell line-derived neurotrophic factor (GDNF) is an endogenous protector in the mesolimbic system against excessive alcohol consumption and relapse. Addict Biol. 2015;20:629–42. doi: 10.1111/adb.12152. [DOI] [PubMed] [Google Scholar]
- Beckers J, et al. Towards better mouse models: enhanced genotypes, systemic phenotyping and envirotype modelling. Nat Rev Genet. 2009;10:371–80. doi: 10.1038/nrg2578. [DOI] [PubMed] [Google Scholar]
- Bespalov MM, et al. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol. 2011;192:153–69. doi: 10.1083/jcb.201009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, et al. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202:336–47. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, et al. Phospholipase C gamma in distinct regions of the ventral tegmental area differentially regulates morphine-induced locomotor activity. Synapse. 2005;56:166–9. doi: 10.1002/syn.20136. [DOI] [PubMed] [Google Scholar]
- Bossert JM, et al. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, et al. Antidepressant treatment outcome depends on the quality of the living environment: a pre-clinical investigation in mice. PLoS ONE. 2013;8:e62226. doi: 10.1371/journal.pone.0062226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–98. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Burke RE. GDNF as a candidate striatal target-derived neurotrophic factor for the development of substantia nigra dopamine neurons. J Neural Transm Suppl. 2006:41–5. doi: 10.1007/978-3-211-45295-0_8. [DOI] [PubMed] [Google Scholar]
- Carnicella S, et al. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–9. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF--a potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, et al. Augmented methamphetamine-induced overflow of striatal dopamine 1 day after GDNF administration. Brain Res. 1999;827:104–12. doi: 10.1016/s0006-8993(99)01314-1. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci. 2009;42:350–62. doi: 10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chapman KM, et al. Targeted Germline Modifications in Rats Using CRISPR/Cas9 and Spermatogonial Stem Cells. Cell Rep. 2015;10:1828–35. doi: 10.1016/j.celrep.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier E, Doudna JA. BIOTECHNOLOGY Rewriting a genome. Nature. 2013;495:50–51. doi: 10.1038/495050a. [DOI] [PubMed] [Google Scholar]
- Cheng AW, et al. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013;23:1163–71. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, et al. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26:907–923. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Codita A, et al. Impaired behavior of female tg-ArcSwe APP mice in the IntelliCage: A longitudinal study. Behav Brain Res. 2010;215:83–94. doi: 10.1016/j.bbr.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Collins FS, et al. A mouse for all reasons. Cell. 2007;128:9–13. doi: 10.1016/j.cell.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, et al. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- Crestani F, et al. Resolving differences in GABAA receptor mutant mouse studies. Nat Neurosci. 2000;3:1059. doi: 10.1038/80553. [DOI] [PubMed] [Google Scholar]
- Domanskyi A, et al. Prospects of Neurotrophic Factors for Parkinson’s Disease: Comparison of Protein and Gene Therapy. Hum Gene Ther. 2015;26:550–9. doi: 10.1089/hum.2015.065. [DOI] [PubMed] [Google Scholar]
- Dreyer JL. New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med. 2010;2:92. doi: 10.1186/gm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper-Mains JE, et al. Global Approaches to the Role of miRNAs in Drug-Induced Changes in Gene Expression. Front Genet. 2012;3:109. doi: 10.3389/fgene.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper-Mains JE, et al. microRNA-Seq reveals cocaine-regulated expression of striatal microRNAs. RNA. 2011;17:1529–43. doi: 10.1261/rna.2775511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, et al. Automated test of behavioral flexibility in mice using a behavioral sequencing task in IntelliCage. Behav Brain Res. 2011;221:172–181. doi: 10.1016/j.bbr.2011.02.037. [DOI] [PubMed] [Google Scholar]
- Ernfors P, et al. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–50. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Faghihi MA, et al. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010;11:R56. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faizi M, et al. Comprehensive behavioral phenotyping of Ts65Dn mouse model of down syndrome: Activation of beta(1)-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiol Dis. 2011;43:397–413. doi: 10.1016/j.nbd.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinato MH, et al. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience. 2015;286:97–108. doi: 10.1016/j.neuroscience.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, et al. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–71. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell. 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Marin A, et al. Big behavioral data: psychology, ethology and the foundations of neuroscience. Nat Neurosci. 2014;17:1455–62. doi: 10.1038/nn.3812. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, et al. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, et al. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, et al. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A. 2005;102:17430–5. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–28. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, Ron D. Autoregulation of glial cell line-derived neurotrophic factor expression: implications for the long-lasting actions of the anti-addiction drug, Ibogaine. FASEB J. 2006;20:2420–2. doi: 10.1096/fj.06-6394fje. [DOI] [PubMed] [Google Scholar]
- Hebert MA, et al. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279:1181–90. [PubMed] [Google Scholar]
- Heikkinen AE, et al. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology. 2009;34:290–8. doi: 10.1038/npp.2008.89. [DOI] [PubMed] [Google Scholar]
- Henriques A, et al. Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: where do we stand? Front Neurosci. 2010;4:32. doi: 10.3389/fnins.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer MP, Kenny PJ. Corticostriatal microRNAs in addiction. Brain Res. 2015 doi: 10.1016/j.brainres.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Hollander JA, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YW, et al. Dual regulation of miRNA biogenesis generates target specificity in neurotrophin-induced protein synthesis. Cell. 2012;148:933–46. doi: 10.1016/j.cell.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–32. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Hunt WA, et al. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–6. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hyman C, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–2. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Ickes BR, et al. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Im HI, et al. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–7. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–34. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izsvak Z, et al. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat Methods. 2010;7:443–5. doi: 10.1038/nmeth.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, et al. RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J Neurosci. 2006;26:11230–8. doi: 10.1523/JNEUROSCI.1876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, et al. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26:1457–64. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, et al. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci. 2013;37:607–12. doi: 10.1111/ejn.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, et al. Genome-wide computational identification and manual annotation of human long noncoding RNA genes. RNA. 2010;16:1478–87. doi: 10.1261/rna.1951310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–62. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–4. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, et al. Reward learning requires activity of matrix metalloproteinase-9 in the central amygdala. J Neurosci. 2013;33:14591–600. doi: 10.1523/JNEUROSCI.5239-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, et al. Differential involvement of the central amygdala in appetitive versus aversive learning. Learn Mem. 2006;13:192–200. doi: 10.1101/lm.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, et al. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front Behav Neurosci. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka W, et al. MicroRNA loss enhances learning and memory in mice. J Neurosci. 2010;30:14835–42. doi: 10.1523/JNEUROSCI.3030-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, et al. BDNF is a negative modulator of morphine action. Science. 2012;338:124–8. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, et al. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat Neurosci. 2015;18:415–22. doi: 10.1038/nn.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopra J, et al. GDNF is not required for catecholaminergic neuron survival in vivo. Nat Neurosci. 2015;18:319–22. doi: 10.1038/nn.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, et al. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, et al. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Krol J, et al. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N, et al. Mixed housing with DBA/2 mice induces stress in C57BL/6 mice: implications for interventions based on social enrichment. Front Behav Neurosci. 2014;8:257. doi: 10.3389/fnbeh.2014.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, et al. GDNF overexpression from the native locus reveals its role in the nigrostriatal dopaminergic system function. PLOS Genetics. 2015 doi: 10.1371/journal.pgen.1005710. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. NCAM signaling mediates the effects of GDNF on chronic morphine-induced neuroadaptations. J Mol Neurosci. 2014;53:580–9. doi: 10.1007/s12031-013-0224-0. [DOI] [PubMed] [Google Scholar]
- Li P, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015;35:8232–44. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LF, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lipp HP, Wolfer DP. Genetically modified mice and cognition. Curr Opin Neurobiol. 1998;8:272–280. doi: 10.1016/s0959-4388(98)80151-7. [DOI] [PubMed] [Google Scholar]
- Lipp HP, Wolfer DP. Genetic background problems in the analysis of cognitive and neuronal changes in genetically modified mice. Clin Neurosci Res. 2003;3:223–231. [Google Scholar]
- Liss B, Roeper J. Individual dopamine midbrain neurons: functional diversity and flexibility in health and disease. Brain Res Rev. 2008;58:314–21. doi: 10.1016/j.brainresrev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Logrip ML, et al. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Logrip ML, et al. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem. 2009;109:1459–68. doi: 10.1111/j.1471-4159.2009.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, et al. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–3. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lu L, et al. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004a;24:1604–11. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, et al. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47(Suppl 1):214–26. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–45. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Luscher C. Synaptic plasticity and addiction: learning mechanisms gone awry. Neuropharmacology. 2011;61:1052–9. doi: 10.1016/j.neuropharm.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Manning EE, et al. BDNF-Deficient Mice Show Reduced Psychosis-Related Behaviors Following Chronic Methamphetamine. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, et al. The Role of BDNF/TrkB Signaling in Acute Amphetamine-Induced Locomotor Activity and Opioid Peptide Gene Expression in the Rat Dorsal Striatum. Front Syst Neurosci. 2011;5:60. doi: 10.3389/fnsys.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, et al. The use of behavioral test batteries: Effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Messer CJ, et al. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–57. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijakowska Z, et al. Autophosphorylation of alpha isoform of calcium/calmodulin-dependent kinase II regulates alcohol addiction-related behaviors. Addict Biol. 2015 doi: 10.1111/adb.12327. [DOI] [PubMed] [Google Scholar]
- Modarresi F, et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat Biotechnol. 2012;30:453–9. doi: 10.1038/nbt.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, et al. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Mucha RF, et al. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, et al. Intrastriatal infusions of brain-derived neurotrophic factor: retrograde transport and colocalization with dopamine containing substantia nigra neurons in rat. Exp Neurol. 1994;129:15–26. doi: 10.1006/exnr.1994.1143. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–28. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Niwa M, et al. The roles of glial cell line-derived neurotrophic factor, tumor necrosis factor-alpha, and an inducer of these factors in drug dependence. J Pharmacol Sci. 2007;104:116–21. doi: 10.1254/jphs.cp0070017. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, et al. Cellular and developmental patterns of expression of Ret and glial cell line-derived neurotrophic factor receptor alpha mRNAs. Exp Brain Res. 1997;115:410–22. doi: 10.1007/pl00005711. [DOI] [PubMed] [Google Scholar]
- Numan S, Seroogy KB. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: a double-label in situ hybridization study. J Comp Neurol. 1999;403:295–308. doi: 10.1002/(sici)1096-9861(19990118)403:3<295::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Oo TF, et al. Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal glial cell line-derived neurotrophic factor in vivo. J Neurosci. 2003;23:5141–8. doi: 10.1523/JNEUROSCI.23-12-05141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratcha G, et al. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–79. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- Parker CC, et al. Rats are the smart choice: Rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology. 2014;76(Pt B):250–8. doi: 10.1016/j.neuropharm.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkitna JR, et al. Novelty-Seeking Behaviors and the Escalation of Alcohol Drinking After Abstinence in Mice Are Controlled by Metabotropic Glutamate Receptor 5 on Neurons Expressing Dopamine D1 Receptors. Biol Psychiatry. 2013;73:263–270. doi: 10.1016/j.biopsych.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Pascual A, et al. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–61. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Patrikainen M, et al. The role of carbonic anhydrase VI in bitter taste perception: evidence from the Car6 −/− mouse model. J Biomed Sci. 2014;21:82. doi: 10.1186/s12929-014-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peregud DI, et al. Expression of BDNF and TrkB Phosphorylation in the Rat Frontal Cortex During Morphine Withdrawal are NO Dependent. Cell Mol Neurobiol. 2015 doi: 10.1007/s10571-015-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- Platt RJ, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–55. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruunsild P, et al. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puscian A, et al. A novel automated behavioral test battery assessing cognitive rigidity in two genetic mouse models of autism. Front Behav Neurosci. 2014;8:140. doi: 10.3389/fnbeh.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Kaczmarek L. Characterization of an alcohol addiction-prone phenotype in mice. Addict Biol. 2012;17:601–612. doi: 10.1111/j.1369-1600.2011.00394.x. [DOI] [PubMed] [Google Scholar]
- Ran FA, et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–9. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy SB, et al. Neurotrophic factor therapy for Parkinson’s disease. Prog Brain Res. 2010;184:237–64. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- Ren Q, et al. 7,8-Dihydroxyflavone, a TrkB agonist, attenuates behavioral abnormalities and neurotoxicity in mice after administration of methamphetamine. Psychopharmacology (Berl) 2014;231:159–66. doi: 10.1007/s00213-013-3221-7. [DOI] [PubMed] [Google Scholar]
- Richardson CA. The power of automated behavioural homecage technologies in characterizing disease progression in laboratory mice: A review. Applied Animal Behaviour Science. 2015;163:19–27. [Google Scholar]
- Ron D, Janak PH. GDNF and addiction. Rev Neurosci. 2005;16:277–85. doi: 10.1515/revneuro.2005.16.4.277. [DOI] [PubMed] [Google Scholar]
- Rosenthal N, Brown S. The mouse ascending: perspectives for human-disease models. Nat Cell Biol. 2007;9:993–9. doi: 10.1038/ncb437. [DOI] [PubMed] [Google Scholar]
- Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–6. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Russo SJ, et al. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D, et al. Spatial learning impairments in PLB1 knock-in Alzheimer mice are task-specific and age-dependent. Cell Mol Life Sci. 2013;70:2603–2619. doi: 10.1007/s00018-013-1314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, et al. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Salmena L, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–8. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–9. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, et al. Neurotrophins: from pathophysiology to treatment in Alzheimer’s disease. Curr Alzheimer Res. 2008;5:38–44. doi: 10.2174/156720508783884620. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, et al. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–34. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Shaham Y, et al. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, et al. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shao Y, et al. CRISPR/Cas-mediated genome editing in the rat via direct injection of one-cell embryos. Nat Protoc. 2014;9:2493–512. doi: 10.1038/nprot.2014.171. [DOI] [PubMed] [Google Scholar]
- Shen F, et al. Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci. 2006;26:11041–51. doi: 10.1523/JNEUROSCI.2898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY, et al. Brain-derived neurotrophic factor increases the electrical activity of pars compacta dopamine neurons in vivo. Proc Natl Acad Sci U S A. 1994;91:8920–4. doi: 10.1073/pnas.91.19.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, et al. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Smutek M, et al. A Model of Alcohol Drinking under an Intermittent Access Schedule Using Group-Housed Mice. PLoS ONE. 2014;9:e96787. doi: 10.1371/journal.pone.0096787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, et al. A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes Brain Behav. 2006;5(Suppl 2):5–24. doi: 10.1111/j.1601-183X.2006.00228.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–7. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, DeVisser L. Advanced behavioural screening: automated home cage ethology. Drug Discovery Today: Technologies. 2006;3:231–237. doi: 10.1016/j.ddtec.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Stragier E, et al. Ethanol-induced epigenetic regulations at the Bdnf gene in C57BL/6J mice. Mol Psychiatry. 2015;20:405–12. doi: 10.1038/mp.2014.38. [DOI] [PubMed] [Google Scholar]
- Sumazin P, et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147:370–81. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33:102–6. doi: 10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–74. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, et al. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci. 2014;34:4581–8. doi: 10.1523/JNEUROSCI.0445-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Nestler EJ. Neurobehavioral assessment in the information age. Nat Neurosci. 2004;7:462–466. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- Tomac A, et al. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A. 1995;92:8274–8. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, et al. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–7. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–88. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31:305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- Wan L, et al. RACK1 affects morphine reward via BDNF. Brain Res. 2011;1416:26–34. doi: 10.1016/j.brainres.2011.07.045. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–8. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannoni E, et al. Spontaneous behavior in the social homecage discriminates strains, lesions and mutations in mice. J Neurosci Methods. 2014;234:26–37. doi: 10.1016/j.jneumeth.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Varendi K, et al. From microRNA target validation to therapy: lessons learned from studies on BDNF. Cell Mol Life Sci. 2015;72:1779–94. doi: 10.1007/s00018-015-1836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–4. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–4. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Veremieva M, et al. Using IntelliCage to study alcohol drinking behavior in mice. Society for Neuroscience meeting; Chicago, IL, USA. October 17–21.2015. [Google Scholar]
- Weyer SW, et al. APP and APLP2 are essential at PNS and CNS synapses for transmission, spatial learning and LTP. EMBO J. 2011;30:2266–2280. doi: 10.1038/emboj.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–6. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Vinnikov IA, et al. Hypothalamic miR-103 protects from hyperphagic obesity in mice. J Neurosci. 2014;34:10659–74. doi: 10.1523/JNEUROSCI.4251-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Voikar V, et al. Conditioned response suppression in the Intellicage: assessment of mouse strain differences and effects of hippocampal and striatal lesions on acquisition and retention of memory. Behav Brain Res. 2010;213:304–312. doi: 10.1016/j.bbr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Voikar V, et al. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Voikar V, et al. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav. 2004;3:27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, et al. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, et al. Efficient gene-targeting in rat embryonic stem cells by CRISPR/Cas and generation of human kynurenine aminotransferase II (KAT II) knock-in rat. Transgenic Res. 2015;24:991–1001. doi: 10.1007/s11248-015-9909-1. [DOI] [PubMed] [Google Scholar]
- Yang H, et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–9. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–23. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, et al. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–60. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]