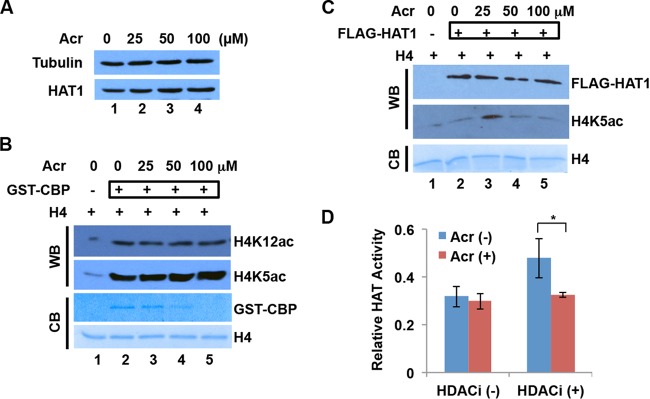
FIG 4.
Effects of Acr exposure on HAT expression and activity. (A) HAT1 protein levels were not changed by Acr exposure in BEAS-2B. BEAS-2B cells were treated with Acr for 2 h. Cytosolic fractions were then extracted for Western blot analysis with HAT1 antibody. Tubulin was used as the loading control. (B) The HAT activity of GST-CBP was not affected by Acr exposure. Recombinant GST-CBP was treated with Acr overnight and then used for a HAT activity assay with recombinant H4 as the substrate. The acetylation reaction was monitored by Western blotting (WB) with H4K5ac and H4K12ac antibodies. Coomassie blue (CB) staining of GST-CBP and H4 is shown. (C) The HAT activity of FLAG-HAT1 was not affected by Acr exposure in vitro. Purified FLAG-HAT1 was treated with Acr overnight and then used for a HAT activity assay with recombinant H4 as the substrate. The FLAG-HAT1 level was determined by Western blotting with FLAG antibody. The acetylation reaction was monitored by Western blotting with H4K5ac antibody. Coomassie blue staining of H4 is shown. (D) The “total” HAT activity of cytosolic fractions was not changed by Acr exposure. After BEAS-2B cells were treated with 100 μM Acr for 2 h or not treated with Acr, cytosolic fractions were isolated in the absence (−) or presence (+) of sodium butyrate, an HDAC inhibitor (HDACi). HAT assays were performed with histone peptides as substrates and cytosolic fractions as an enzyme source. The average values ± SD of three independent experiments are presented. *, P < 0.05.