Figure 5. EBF1 and EBF2 physically interact with COP1 in vitro and in vivo.
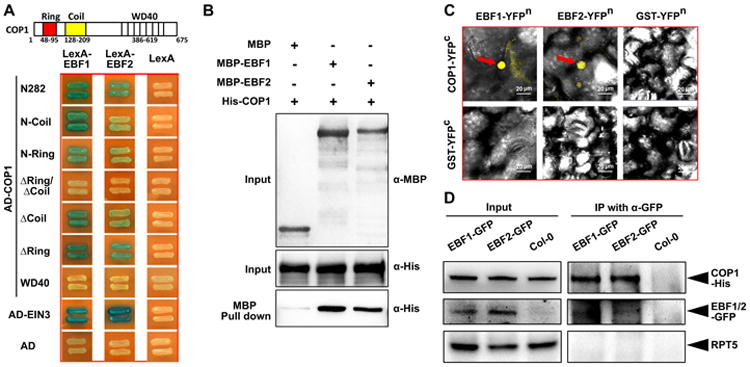
(A) N-terminal fragments of COP1 directly interact with EBF1 and EBF2 in yeast two-hybrid assays. Left diagrams indicate the various fragments of COP1 fused with the activation domain. Full length EBF1 and EBF2 fused with LexA DNA binding domain were the prey constructs in the assay.
(B) COP1 and EBF1/2 directly interact in pull-down assays. Purified COP1-His was used as prey and was pulled down by the baits EBF1-MBP, EBF2-MBP and MBP, respectively. Anti-MBP and anti-His were used for the immunoblot analysis.
(C) BiFC assay reveals that COP1 interacts with EBF1 and EBF2 in the nucleus of Nicotiana benthamiana leaf cells. Full length COP1 or EBF1 and EBF2 were fused to the split N-terminal or C-terminal (YFPn or YFPc) fragments of YFP. GST fused to YFPn or YFPc fragments were used as negative controls. Red arrow indicates the position of YFP speckles. Bar = 20 μm.
(D) Semi-in vivo co-immunoprecipitation assay of COP1 with EBF1 and EBF2 proteins. EBF1-GFP and EBF2-GFP overexpression transgenic plants and Col-0 control were grown in the dark for 4 days. Equal amount of COP1-His proteins were added into the cell extracts and immunoprecipitated using anti-GFP antibody and immunoblotted using indicated antibodies.
