Abstract
Wings were a fundamental morphological innovation for the adaptive radiation of insects, the most diversified group among all animals. Pterygote insects have two pairs of wings, the mesothoracic (T2) forewings and the metathoracic (T3) hindwings, whereas the prothorax (T1) is wingless. Using RNA interference approaches, we have found that the gene Sex combs reduced (Scr) determines the wingless identity of T1 in the cockroach Blattella germanica. Interference of Scr triggers the formation of ectopic wing structures in T1, which are formed from the expansion of the latero-posterior region of the pronotum, along with a contribution of the epimeron, a pleurite of T1. These data support the theory of a dual origin for insect wings, from pronotal (tergal origin theory) and pleural (pleural origin theory) structures and genes.
Keywords: origin insect wings, sex combs reduced, Blattella, Drosophila, Tribolium
1. Introduction
Wings were a morpho-functional innovation crucial for the spectacular radiation of insects, the most speciose lineage among all animals. Present winged insects, the Pterygota, have two pairs of wings located on the second (T2) and third (T3) thoracic segments, whereas the first thoracic segment (T1) is wingless [1]. Wing morphogenesis in T2 and T3 is regulated by a complex gene cascade [2], whereas the wingless identity of T1 mainly results from the action of the Hox gene Sex combs reduced (Scr). The first data suggesting that Scr might repress wing formation in T1 came from the work of Carroll et al. [3], who showed that it inhibits the expression of vestigial (vg) and snail (two genes required for wing formation) in the primordia of dorsal discs of the fruit fly, Drosophila melanogaster. Two years later, Rogers et al. [4] reported that D. melanogaster carrying a hypomorphic heterozygous allele with a null allele for Scr formed wing tissue in T1. Similar experiments carried out with the Scr orthologue Cephalothorax in the flour beetle, Tribolium castaneum, elicited the formation of elytra in T1 [5], a phenotype that was also observed when depleting Scr expression by RNA interference (RNAi) [6]. More recently, formation of wing-like tissue in T1 after RNAi of Scr has been reported in the large milkweed bug, Oncopeltus fasciatus [7,8], and the American cockroach, Periplaneta americana [9].
The origin of insect wings has been the object of continuous interest (and considerable controversy), and has led to two basic theories (see [10] for a recent review). One theory proposes that wings originated from an extension of the thoracic tergum, first forming paranotal lobes and then fully articulated wings. This tergal origin theory was initially suggested by F. Müller in 1875, formalized by Crampton [11] and supported by Snodgrass [12] and Hamilton [13] (see [14]). The theory of tergal origin has also been invoked in modern developmental studies based on molecular approaches [9,15].
The second theory considers the wing is derived from pleural structures and also has very ancient origins, dating back to Oken [16] and Woodworth [17]. In modern times, it has been championed by Kukalová-Peck [18–21], who proposed the wing derives from articulated exites that were located on the proximal leg segments of ancestral insects that migrated dorsally and finally formed the articulated and flying wing. An influential paper by Averof & Cohen [22] reported that insect wing-related genes are expressed in dorsal gills of crustacean branched limbs, providing support for the pleural origin theory. Recent morphological and molecular investigations have shown that the embryonic subcoxa contribute to form pleural sclerites of the adult [23] and have also provided significant arguments in favour of the pleural origin theory.
In the past, the theory of tergal origin has proved the most popular, possibly because it appears coherent with the flat form and position of modern wings; however, it does not account for the complex musculature and articulations needed for flight functions. Conversely, the theory of pleural origin explains the origin of muscles and articulations as they are already contained in the leg branch limb, but this hypothesis is counterintuitive when trying to imagine how a leg branch can transform into a flattened and flexible structure like the wing of a flying insect. A possible solution to this disparity might be to merge both theories, thus explaining the origin of wings according to a dual contribution of tergal and pleural structures. Indeed this eclectic approach has a historical background as it was first suggested by Crampton [11], although he clearly favoured the tergal origin theory. More recently, Rasnitsyn [24] modified the tergal origin theory of wings by adding the contribution of proximal leg segments. However, the most convincing data pointing to wings having a dual origin, combining tergal and pleural contributions, have been produced recently following molecular approaches by the groups of Hayashi and co-workers [25] and Tomoyasu and co-workers [26].
The Hayashi group used the bristletail Pedetontus unimaculatus (apterygota, Archaeognatha) and the mayfly Ephoron eophilum (Paleoptera, Ephemeroptera) as experimental subjects (both of which develop dorsal limb branches, styli and tracheal gills, respectively) and studied the expression of three genes key to insect wing development, wingless (wg), vestigial (vg) and apterous (ap). Results showed that wg and vg are expressed in the primordia of styli and tracheal gills, whereas the lateral tergal margin expresses these two genes in addition to ap. The authors concluded that insect wings might have originated from the fusion of the modules corresponding to the tergal margin and to the limb branch [25].
The Tomoyasu research group used T. castaneum (Endopterygota, Coleoptera) as model and RNAi experiments, and found that the lateral tergal margin (designated by these authors as the carinated margin) as well as some pleural plates in T1 are vg dependent [26]. Interestingly, in T2 and T3 vg functions in the wings, and systemic interference of Scr, a gene that determines the wingless identity of T1, triggers the homeotic transformation of T1 into T2 including the formation of ectopic wings in T1 through the contribution of those T1 structures where vg is expressed [6,26]. These data provide compelling support for the theory that incorporates a dual origin of wings, with tergal and pleural contributions.
A most recent paper by Popadić and co-workers, using the hemimetabolan species, O. fasciatus (Condylognatha, Hemiptera) as model, reports that the T1 wing structures formed after Scr depletion in this species are mainly of dorsal origin, although gene expression differences between the ectopic T1 wing structure and T2 and T3 wings suggest that ventral structures also contribute to wing formation [8]. These results would also support, thus, a dual origin for insect wings.
This work aims to provide a contribution in this context by studying the changes on T1 triggered by Scr depletion using the German cockroach Blattella germanica as the experimental subject. Blattella germanica is a hemimetabolan species like O. fasciatus, but belongs to the subclass Polyneoptera, the sister group of Condylognatha + Psocodea + Endopterygota (=Holometabola) [27].
2. Material and methods
2.1. Insects
Blattella germanica specimens used in the experiments were obtained from a colony reared in the dark at 30 ± 1°C and 60–70% RH. They were carbon dioxide-anaesthetized prior to dissections and tissue sampling.
2.2. RNA extraction and retrotranscription to cDNA
All RNA extractions were carried out with the Gen Elute Mammalian Total RNA kit (Sigma-Aldrich, Madrid, Spain). An amount of 400 ng from each RNA extraction was treated with DNase (Promega, Madison, WI, USA) and reverse transcribed with Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and random hexamers (Promega). RNA quantity and quality were estimated by spectrophotometric absorption at 260 nm in a Nanodrop spectrophotometer ND-1000® (NanoDrop Technologies, Wilmington, DE, USA).
2.3. Determination of mRNA levels with quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) reactions were carried out in triplicate in an iQ5 real-time PCR detection system (Bio-Rad Laboratories, Madrid, Spain), using SYBR®Green (Power SYBR® Green PCR Master Mix; Applied Biosystems, Madrid, Spain). A control without template was included in all batches. In addition to measuring mRNA levels of Scr, we also measured the expression of the following 16 genes involved in wing formation, whose sequence was available in the B. germanica transcriptome of metanotum epidermis obtained in our laboratory (GEO accession number GSM1569374): apterous a (ap-a), blistered (bs), cut (ct), delta (Dl), decapentaplegic (dpp), epidermal growth factor receptor (Egfr), engrailed (en), notch (N), nubbin (nub), rhomboid (rho), serrate (Ser), scalloped (sd), spalt (salm), ultrabithorax (Ubx), vestigial (vg) and wingless (wg). The primers used for each transcript measured are detailed in the electronic supplementary material, table S1. The efficiency of each primer set was first validated by constructing a standard curve through four serial dilutions. mRNA levels were quantified relative to BgActin-5c expression if not stated otherwise, using the Bio-Rad iQ5 Standard Edition Optical System Software (v. 2.0); primers are indicated in the electronic supplementary material table S1. We followed a method based on Ct (threshold-cycle) according to the Pfaffl mathematical model [28], simplifying to 2ΔΔCt because the calculated efficiency values for studied genes and BgActin-5c amplicons were always within the range of 95–100%; therefore, no correction for efficiency was used in further calculations. Results are given as copies of mRNA per 1000 copies of BgActin-5c mRNA.
2.4. RNA interference
In general, we followed the RNAi approach previously described [29]. The primers to generate the templates for the dsRNA targeting Scr (dsScr) are indicated in the electronic supplementary material, table S1. The fragments were amplified by PCR and cloned into the pSTBlue-1 vector (Novagen, Madrid, Spain). In all cases, we used a 307 bp sequence from Autographa californica nucleopolyhydrosis virus (accession no. K01149, from nucleotide 370 to 676) as control dsRNA (dsMock). The dsRNAs were prepared as reported elsewhere [29]. Female nymphs were treated with three 2 µg doses of dsScr in 1 µl volume injected into the abdomen with a 5 µl Hamilton microsyringe, the first in freshly emerged fourth instar, the second in freshly emerged fifth instar and the third in freshly emerged sixth instar (n = 51). Control specimens were equivalently treated with dsMock (n = 23). Transcript decrease was examined by qRT-PCR in T1 on day 6 of the sixth nymphal instar.
2.5. Morphological studies
Routine examinations and photographs were made with an optic stereomicroscope Zeiss DiscoveryV8. For scanning electron microscopy (SEM), the specimens were dried at room temperature and glued to stubs with colloidal silver, sputter coated with gold palladium, and examined with a Hitachi S-3500N (Nissei Sangyo Co. Ltd., Tokyo, Japan) SEM operating at 5 kV. Integument preparations were briefly rinsed with Ringer saline (NaCl 0.17 M; KCl 0.01 M; CaCl2 0.003 M), and kept for 12 h in cold (4°C) fixative (4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.3). Next, integuments were dehydrated in a graded ethanol series (70%, 80%, 90% and 95% ethanol in water, v/v) for 15 min each, followed by two rinses in 100% for 15 min each. Samples were critical point-dried in liquid CO2 using a BAL-TEC CPD 030 critical point drying apparatus (Balzers Union, Balzers, Germany).
2.6. Statistics
Quantitative data are expressed as mean ± s.e.m. In qRT-PCR determinations, statistical analyses between groups were tested by the REST 2008 program (Relative Expression Software Tool V 2.0.7; Corbett Research) [28]. This program makes no assumptions about the distributions, evaluating the significance of the derived results by pair-wise fixed reallocation randomization test tool.
3. Results
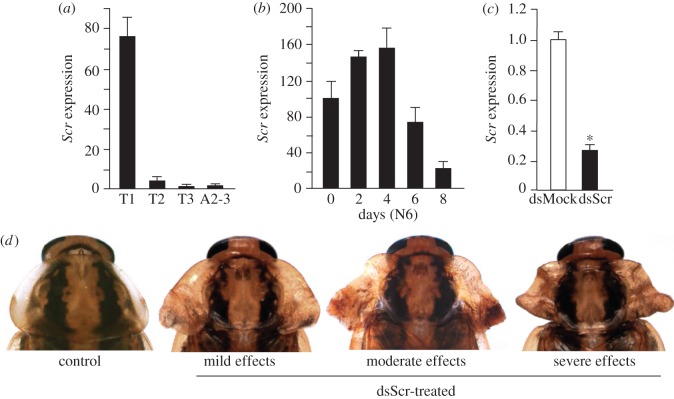
Scr is significantly expressed only in T1 (figure 1a). In the last instar nymph, expression follows a pattern of gradual increase until day 4, and then a rapid decrease during days 6–8 (figure 1b). We depleted Scr mRNA levels in female nymphs following an RNAi approach, applying three 2 µg doses of dsScr (treated) or dsMock (controls), the first injected in freshly emerged fourth instar (N4D0), the second in N5D0 and the third in N6D0. Transcript decrease was examined in T1 on N6D6, when the moulting peak of ecdysone is being produced [30] and wing maturation proceeds in T2 and T3 [31]. Results showed that RNAi efficiently reduced Scr expression (approx. 75% Scr mRNA reduction; figure 1c).
Figure 1.
Expression of Scr in Blattella germanica and RNAi effects. (a) mRNA levels of Scr in the three thoracic segments, prothorax (T1), mesothorax (T2) and metathorax (T3), and in the 2nd and 3rd abdominal segments (A2–3) of female nymphs, measured on day 6 of sixth (last) instar (N6D6). (b) mRNA levels of Scr during N6 in T1. (c) mRNA levels of Scr measured in T1 on N6D6 in controls (dsMock-treated) and dsScr-treated insects; female nymphs were treated with three 2 µg doses of dsRNA, the first injected in freshly emerged fourth instar (N4D0), the second in N5D0 and the third in N6D0. (d) Dorsal view of the pronotum of adult females from the control (dsMock-treated, n = 23) and dsScr-treated groups (n = 51), including examples of mild, moderate and severe effects. Expression (a–c) is represented as mRNA copies per 1000 copies of actin-5c (mean ± s.e.m., n = 4). In (c), expression is normalized against respective control values (indicated as 1) and the asterisk indicates statistically significant differences with respect to controls (p < 0.05), according to REST [28].
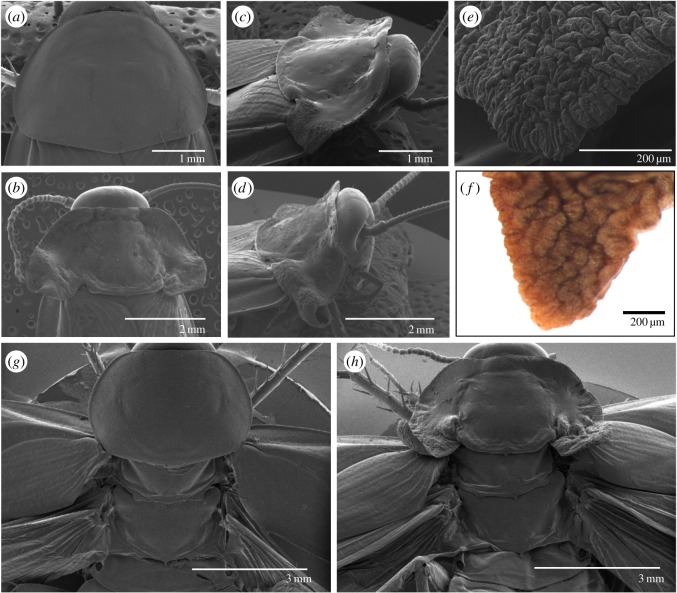
DsScr-treated nymphs (n = 51) moulted to N5, to N6 and then to adults as in dsMock-treated controls (n = 23). However, the adults emerging from dsScr-treated nymphs showed conspicuous latero-posterior expansions of the pronotal edge, from mild to moderate or severe (figure 1d). In mild phenotypes, the pronotum presented a similar shape to the controls, although slightly more transverse (in controls, the pronotum was approximately as long as it was wide, figures 1d and 2a), with the latero-posterior region slightly expanded, especially near the base, forming a lobular structure with a slightly wrinkled surface (figure 1d). Moderate phenotypes had a clearly transverse pronotum, the latero-posterior region was more expanded, and the lobular basal structure notably wrinkled (figure 1d). Moreover, the pronotum, which was uniformly convex in the controls (figures 1d and 2a), showed two longitudinal grooves separating the actual pronotal disc from the latero-posterior expansions, which ended at the base with respective notches. The anterior and posterior edges each revealed very marked transverse grooves delimiting the central part of the pronotal disc, whose length was greater than its width and was almost flat. Finally, in the centre of the posterior edge, there was a small, backward-facing, triangular protrusion (figures 1d and 2b,c). The specimens showing the most severe effects were essentially similar, but their latero-posterior expansions were even more developed, especially the lobular basal structure, which was larger, heavily wrinkled and folded, and adopted curled morphologies (figures 1d and 2d). At high magnification, the wrinkled lobular structure of these specimens (figure 2e) was similar to the wing bud that develops in the pocket of the latero-posterior area of T2 and T3 observed on day 6 of the last nymphal instar [31], giving rise to the tegmina and membranous wings, respectively, after the imaginal moult (figure 2f).
Figure 2.
SEM examinations of the effects of Scr depletion in Blattella germanica on the thorax dorsal region. (a) Dorsal view of T1 of a control adult. (b) Dorsal view of T1 of an adult obtained after dsScr treatments, with moderately developed lateral expansions (moderate phenotype). (c) Dorso-lateral view of T1 of an adult obtained after dsScr treatments, with moderately developed lateral expansions (moderate phenotype). (d) Dorso-lateral view of T1 of an adult obtained after dsScr treatments, with conspicuously developed lateral expansions (severe phenotype). (e) Detail of the tip of the right lateral expansion shown in (c). (f) Tip of the wing bud located in the pocket of T2 lateral expansions on day 6 of the last nymphal instar. (g,h) Dorsal view of T1, T2 and T3 of a control adult (g) and an adult obtained following dsScr treatments (h) with the T2 and T3 wings extended.
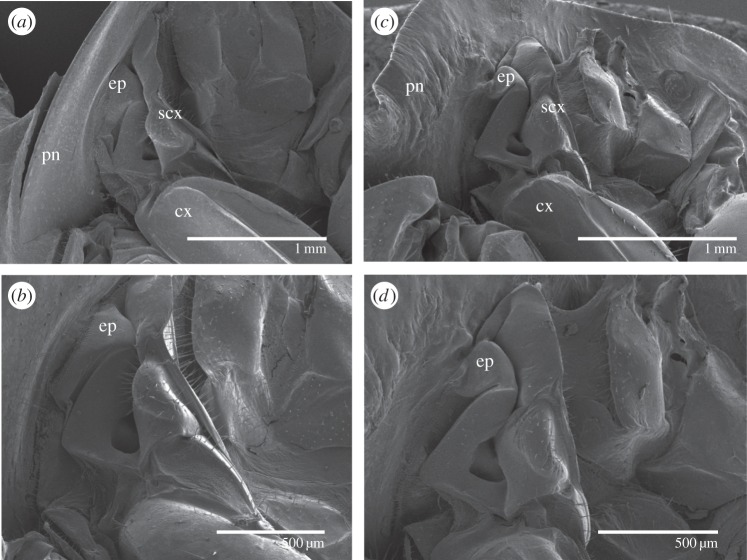
Examination of the dorsal view of T1, T2 and T3 in controls (figure 2g) and in Scr-depleted specimens (figure 2h) revealed that the morphology of T1 in the latter group resembles that of T2, especially in terms of the presence of the transverse groove close to the posterior edge and the small, backward-facing, triangular protrusion in the centre of the edge in T1 of Scr-depleted specimens. Examination in latero-ventral view showed that the pleural area of T1 did not appear to be significantly modified in Scr-depleted specimens, except for the epimeron, a sclerite proximal to the subcoxal region, which was reduced and fused to the wing-like latero-posterior expansions (figure 3a–d), suggesting that this pleurite contributed to the formation of these expansions.
Figure 3.
SEM examinations of the effects of Scr depletion in Blattella germanica on the prothorax pleural region. (a,b) Lateral view of T1 of a control adult female at two magnifications. (c,d) Lateral view of T1 of an adult female obtained after dsScr treatments at two magnifications. ep, epimeron; cx, coxa; scx, subcoxa; pn, hypomeral area of the pronotum.
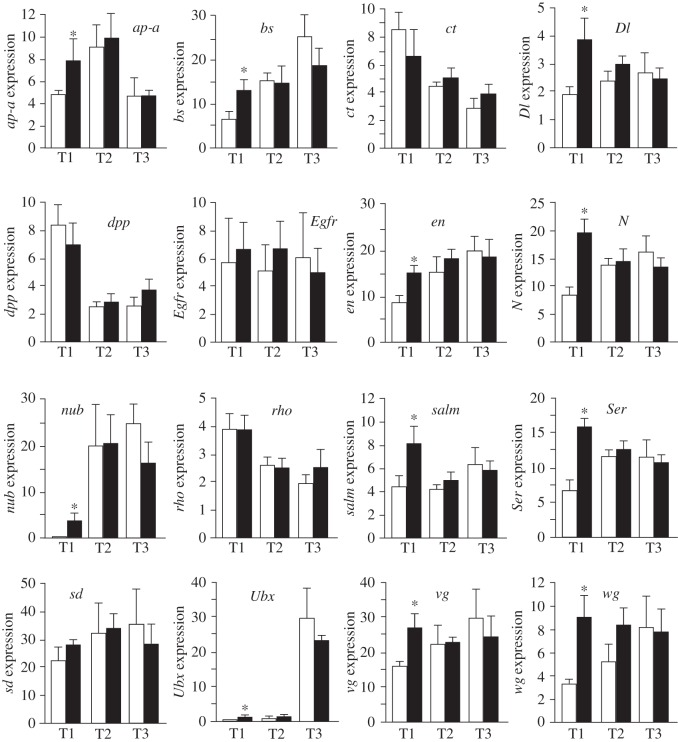
Finally, we examined the expression levels of 16 genes involved in wing development. Interference of Scr resulted in a significant increase of expression of 11 of these genes in T1: ap-a, bs, Dl, en, N, nub, Ser, salm, Ubx, vg and wg. Only ct, dpp, Egfr, rho and sd were not significantly affected in T1, whereas Scr depletion had no significant effect on the expression of the 16 genes in T2 and T3 (figure 4). Figure S1 in the electronic supplementary material is a schematic of the relative expression of the 16 genes in control T1, T2 and T3, and in T1 from dsScr-treated specimens.
Figure 4.
Effect of Scr depletion on the expression of a series of genes involved in wing development in T1, T2 and T3. ap-a, apterous a; bs, blistered; ct, cut; Dl, delta; dpp, decapentaplegic; Egfr, epidermal growth factor receptor; en, engrailed; N, notch; nub, nubbin; rho, rhomboid; salm, spalt; Ser, serrate; sd, scalloped; Ubx, ultrabithorax; vg, vestigial; wg, wingless. Expression is represented as mRNA copies per 1000 copies of actin-5c (mean ± s.e.m., n = 4). The asterisk indicates statistically significant differences with respect to controls (p < 0.05), according to REST [28].
4. Discussion
In B. germanica, Scr depletion triggered the formation of hardened and deeply folded wing-like expansions in the latero-posterior region of T1, exactly where wing pads locate in T2 and T3 [31]. Before adult ecdysis the wing pads show a deeply folded morphology [31] very similar to that of the T1 wing-like structures formed after Scr depletion. The study of the expression of a number of wing-related genes in T1 wing-like structures showed that 11 out of the 16 genes studied, including ap-a, nub, vg and wg, were significantly upregulated. This indicates that at least these 11 wing-related genes are directly or indirectly repressed by Scr in T1. The T1 wing-like structures obtained in our experiments with B. germanica are similar to those reported in P. americana, after Scr depletion [9] and are reminiscent of those characterizing the Prowing (Pw) homeotic mutant of B. germanica, first described by Ross [32] and possibly associated with a breakage of chromosome 9 [33]. More recent studies [34] suggest that a number of different factors may be involved in the expression of Pw, but the characteristic phenotype suggests that the Scr gene may have been affected.
Besides the wing-like expansions, the general dorsal morphology of T1 in Scr-depleted specimens is reminiscent of T2, especially because of the occurrence of a transverse groove close to the posterior edge and a triangular protrusion in this edge, which are typical structures of T2. This resemblance of dorsal morphology between T1 of Scr-depleted specimens and T2 has been also observed in P. americana [9], as well as in O. fasciatus, where the formation of an ectopic scutellum (which is typical of T2) in T1 is well apparent [7,8], which suggested to the respective authors that Scr depletion triggered a partial transformation of T1 to T2.
In Scr-depleted specimens we observed that the epimeron, a sclerite located in the proximal area of the T1 pleural region, was smaller than in control specimens and fused to the latero-posterior expansion of the pronotum, which formed the bulk of the wing-like structures, thus suggesting that this pleurite contributed to their formation. Prothoracic latero-ventral structures were not studied in Scr-depleted P. americana [9], but in the case of O. fasciatus the epimeron was visibly reduced in size and the shape changed from triangular to somewhat rectangular in Scr-depleted specimens [7]. Similar effects have been also observed in T. castaneum, in which the base of the epimeron expands and fuses to the ectopic elytra formed in T1 in the adult of Scr-depleted specimens [26]. As pointed out by Clark-Hachtel et al. [26], the contribution of pleural structures to the formation of the ectopic wing in T1 of Scr-depleted T. castaneum clearly favoured the hypothesis that insect wings have a dual (tergal and pleural) origin. The results presented herein and those reported in O. fasciatus [7] show that the contribution of the epimeron to the formation of the T1 ectopic wing is conserved from Polyneoptera (B. germanica) to Condylognatha (O. fasciatus) and Endopterygota (T. castaneum), which lends further robustness to the hypothesis proposing that insect wings have a tergal and pleural origin.
It has been previously stressed [8] that in the endopterygotes D. melanogaster [4] and T. castaneum [5,6] Scr depletion triggers a complete homeotic transformation of T1 into T2, whereas in O. fasciatus [7,8] the effects mainly concern the dorsal part, including the ectopic wings, which have been considered a unique structure [8]. The present study shows that the effects of Scr depletion in the polyneopteran B. germanica are similar to those observed in the condylognathan O. fasciatus. Indeed, the whole data suggest that the difference between complete and partial transformation of T1 to T2 possibly derives from the different mode of metamorphosis, hemimetabolan in B. germanica and O. fasciatus, and holometabolan in T. castaneum and D. melanogaster. In the hemimetabolan mode, the juvenile nymphs already have the basic adult body plan, and the successive nymphal moults allow growth with little morphological change; the main changes occur during the imaginal moult, when wings and genitalia are completely formed [35]. Thus, the establishment of the basic adult body plan in the hemimetabolan embryo might preclude a global segment transformation in the postembryonic period, as occurs in T1 when Scr is interfered with, and only a partial transformation is triggered at the imaginal moult, affecting the wings and adjacent dorsal structures and, interestingly, discrete pleural sclerites associated with wing formation. Conversely, in the holometabolan mode the adult body plan is formed in the pupal stage, essentially from a series of cells destined to develop into specific adult structures, which in D. melanogaster are grouped into specific imaginal discs, and that are carried during the larval stages [35,36]. Under this developmental scenario, Scr depletion in larval stages would trigger the global transformation of T1 to T2 in the pupae.
What seems clear is that the mechanism by which the Hox gene Scr confers the wingless identity of T1 is conserved from cockroaches to flies. Insect wings originated in early Devonian, with the emergence of pterygotes [27]. Then, pterygotes underwent a major radiation in the Carboniferous, where a number of groups, for example within the palaeodictyopterans, meganisopterans, protorthopterans, typically had two pairs of fully developed wings in T2 and T3, and a pair of lateral lobes or winglets on the T1 tergum [1]. However, no fossils are known with fully developed wings in T1, suggesting that co-option of Scr to repress the formation of full wings in T1 was an early event in wing evolution. Interestingly, the firebrat Thermobia domestica (apterygota, Zygentoma), a primitively wingless insect, expresses Scr in the prothoracic dorsum [4], thus predating the emergence of specific functions of wing formation repression that operate in the pterygotes. Therefore, the tool that would facilitate the suppression of wings in T1 was already available for co-option in apterygotes.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Cristina Olivella and José Fortuño for technical assistance. We also thank Jarmila Kukalová-Peck, Yoshinori Tomoyasu, Courtney M. Clark-Hatchel, Jim Marden, Maria-Dolors Piulachs, José Luis Maestro, Dalton Amorim and Márcia Bitondi for helpful suggestions on the project and on the manuscript.
Ethics
Rearing and manipulations of Blattella germanica specimens were carried out in accordance with the regulations of the CSIC Committee of Bioethics.
Data accessibility
The sequences of all wing genes studied in this work were obtained from the Blattella germanica transcriptome of metanotum epidermis, available at the Gene Expression Omnibus (GEO) database (accession no. GSM1569374). In addition, the GenBank accession number of each gene is indicated in electronic supplementary material, table S1. Sequences of primers used for mRNA quantification by qRT-PCR and for dsRNA preparation are also indicated in the electronic supplementary material, table S1.
Authors' contributions
M.E.-N. performed experiments, contributed to the experimental design and data analysis, and wrote the manuscript. X.B. conceived and supervised the project, contributed to experimental design and data analysis, and edited the final manuscript, with contributions and approval from M.E.N.
Competing interests
We have no competing interests concerning this work.
Funding
Support for this research was provided to X.B. by the Spanish Ministry of Science and Innovation (grant no. CGL2008-03517/BOS) and Ministry of Economy and Competitiveness (grant CGL2012-36251 and CGL2015-64727- P, including FEDER funds) and by the Catalan Government, AGAUR (2014 SGR 619). M.E.N. was awarded a post-doctoral fellowship by the Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP 2012/15397-0).
References
- 1.Grimaldi D, Engel MS. 2005. Evolution of the insects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Brook WJ, Diaz-Benjumea FJ, Cohen SM. 1996. Organizing spatial pattern in limb development. Annu. Rev. Cell Dev. Biol. 12, 161–180. (doi:10.1146/annurev.cellbio.12.1.161) [DOI] [PubMed] [Google Scholar]
- 3.Carroll SB, Weatherbee SD, Langeland JA. 1995. Homeotic genes and the regulation and evolution of insect wing number. Nature 375, 58–61. (doi:10.1038/375058a0) [DOI] [PubMed] [Google Scholar]
- 4.Rogers BT, Peterson MD, Kaufman TC. 1997. Evolution of the insect body plan as revealed by the sex combs reduced expression pattern. Development 124, 149–157. [DOI] [PubMed] [Google Scholar]
- 5.Beeman RW, Stuart JJ, Haas MS, Denell RE. 1989. Genetic analysis of the homeotic gene complex (HOM-C) in the beetle Tribolium castaneum. Dev. Biol. 133, 196–209. (doi:10.1016/0012-1606(89)90311-4) [DOI] [PubMed] [Google Scholar]
- 6.Tomoyasu Y, Wheeler SR, Denell RE. 2005. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature 433, 643–647. (doi:10.1038/nature03272) [DOI] [PubMed] [Google Scholar]
- 7.Chesebro J, Hrycaj S, Mahfooz N, Popadic A. 2009. Diverging functions of Scr between embryonic and post-embryonic development in a hemimetabolous insect, Oncopeltus fasciatus. Dev. Biol. 329, 142–151. (doi:10.1016/j.ydbio.2009.01.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medved V, Marden JH, Fescemyer HW, Der JP, Liu J, Mahfooz N, Popadic A. 2015. Origin and diversification of wings: insights from a neopteran insect. Proc. Natl Acad. Sci USA 112, 15 946–15 951. (doi:10.1073/pnas.1509517112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrycaj S, Chesebro J, Popadic A. 2010. Functional analysis of Scr during embryonic and post-embryonic development in the cockroach, Periplaneta americana. Dev. Biol. 341, 324–334. (doi:10.1016/j.ydbio.2010.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark-Hachtel CM, Tomoyasu Y. 2016. Exploring the origin of insect wings from an evo-devo perspective. Curr. Opin. Insect Sci. 13, 77–85. (doi:10.1016/j.cois.2015.12.005) [DOI] [PubMed] [Google Scholar]
- 11.Crampton G. 1916. The phylogenetic origin and the nature of the wings of insects according to the paranotal theory. J. N Y Entomol. Soc. 24, 1–39. [Google Scholar]
- 12.Snodgrass RE. 1935. Principles of insect morphology. New York, NY: McGraw-Hill Book Company. [Google Scholar]
- 13.Hamilton KGA. 1971. The insect wing. Part 1. Origin and development of wings from notal lobes. J. Kansas Entomol. Soc. 44, 421–433. [Google Scholar]
- 14.Quartau JA. 1986. An overview of the paranotal theory on the origin of the insect wings. Publicações do Instituto de Zoologia ‘Dr Augusto Nobre’, vol. 194, pp. 1–42. Faculdade de Ciencias do Porto. [Google Scholar]
- 15.Ohde T, Yaginuma T, Niimi T. 2013. Insect morphological diversification through the modification of wing serial homologs. Science 340, 495–498. (doi:10.1126/science.1234219) [DOI] [PubMed] [Google Scholar]
- 16.Oken L. 1809–1811 Lehrbuch der Naturphilosophie. 3 vols Jena: Friedrich Frommann. [Google Scholar]
- 17.Woodworth CW. 1906. The wing veins of insects. Univ. Calif. Publ. Tech. Bull. 1, 1–152. [Google Scholar]
- 18.Kukalova-Peck J. 1978. Origin and evolution of insect wings and their relation to metamorphosis, as documented by the fossil record. J. Morph. 156, 53–126. (doi:10.1002/jmor.1051560104) [DOI] [PubMed] [Google Scholar]
- 19.Kukalova-Peck J. 1983. Origin of the insect wing and wing articulation from the arthropodan leg. Can. J. Zool. 61, 1618–1669. (doi:10.1139/z83-217) [Google Scholar]
- 20.Kukalova-Peck J. 1991. Fossil history and the evolution of hexapod structures. In The insects of Australia: a textbook for students and research workers (ed. Naumann ID.), pp. 141–179. New York, NY: Cornell University Press. [Google Scholar]
- 21.Kukalova-Peck J. 2008. Phylogeny of higher taxa in insecta: finding synapomorphies in the extant fauna and separating them from homoplasies. Evol. Biol. 35, 4–51. (doi:10.1007/s11692-007-9013-4) [Google Scholar]
- 22.Averof M, Cohen SM. 1997. Evolutionary origin of insect wings from ancestral gills. Nature 385, 627–630. (doi:10.1038/385627a0) [DOI] [PubMed] [Google Scholar]
- 23.Coulcher JF, Edgecombe GD, Telford MJ. 2015. Molecular developmental evidence for a subcoxal origin of pleurites in insects and identity of the subcoxa in the gnathal appendages. Sci. Rep. 5, 15757 (doi:10.1038/srep15757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasnitsyn AP. 1981. A modified paranotal theory of the insect wing origin. J. Morphol. 168, 331–338. (doi:10.1002/jmor.1051680309) [DOI] [PubMed] [Google Scholar]
- 25.Niwa N, Akimoto-Kato A, Niimi T, Tojo K, Machida R, Hayashi S. 2010. Evolutionary origin of the insect wing via integration of two developmental modules. Evol. Dev. 12, 168–176. (doi:10.1111/j.1525-142X.2010.00402.x) [DOI] [PubMed] [Google Scholar]
- 26.Clark-Hachtel CM, Linz DM, Tomoyasu Y. 2013. Insights into insect wing origin provided by functional analysis of vestigial in the red flour beetle, Tribolium castaneum. Proc. Natl. Acad. Sci USA 110, 16 951–16 956. (doi:10.1073/pnas.1304332110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. (doi:10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30, e36 (doi:10.1093/nar/30.9.e36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciudad L, Piulachs MD, Belles X. 2006. Systemic RNAi of the cockroach vitellogenin receptor results in a phenotype similar to that of the Drosophila yolkless mutant. FEBS J. 273, 325–335. (doi:10.1111/j.1742-4658.2005.05066.x) [DOI] [PubMed] [Google Scholar]
- 30.Romaña I, Pascual N, Belles X. 1995. The ovary is a source of circulating ecdysteroids in Blattella germanica (L.) (Dictyoptera, Blattellidae). Eur. J. Entomol. 92, 93–103. [Google Scholar]
- 31.Huang JH, Lozano J, Belles X. 2013. Broad-complex functions in postembryonic development of the cockroach Blattella germanica shed new light on the evolution of insect metamorphosis. Biochim. Biophys. Acta 1830, 2178–2187. (doi:10.1016/j.bbagen.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 32.Ross MH. 1964. Pronotal wings in Blattella germanica (L.) and their possible evolutionary significance. Am. Mid. Nat. 71, 161–180. (doi:10.2307/2422693) [Google Scholar]
- 33.Ross MH, Cochran DG. 1971. Cytology and genetics of a pronotal-wing trait in the German cockroach. Can. J. Genet. Cytol. 13, 522–535. (doi:10.1139/g71-078) [DOI] [PubMed] [Google Scholar]
- 34.Tanaka A, Ito T. 1997. Studies of the genetics and expression of Prowing (Pw): a primitive homeotic mutant of the German cockroach, Blattella germanica. Zool. Sci. 14, 339–346. (doi:10.2108/zsj.14.339) [Google Scholar]
- 35.Belles X. 2011. Origin and evolution of insect metamorphosis. In Encyclopedy of life sciences (ELS), pp. 1–11. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 36.Svacha P. 1992. What are and what are not imaginal discs: reevaluation of some basic concepts (Insecta, Holometabola). Dev. Biol. 154, 101–117. (doi:10.1016/0012-1606(92)90052-I) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences of all wing genes studied in this work were obtained from the Blattella germanica transcriptome of metanotum epidermis, available at the Gene Expression Omnibus (GEO) database (accession no. GSM1569374). In addition, the GenBank accession number of each gene is indicated in electronic supplementary material, table S1. Sequences of primers used for mRNA quantification by qRT-PCR and for dsRNA preparation are also indicated in the electronic supplementary material, table S1.