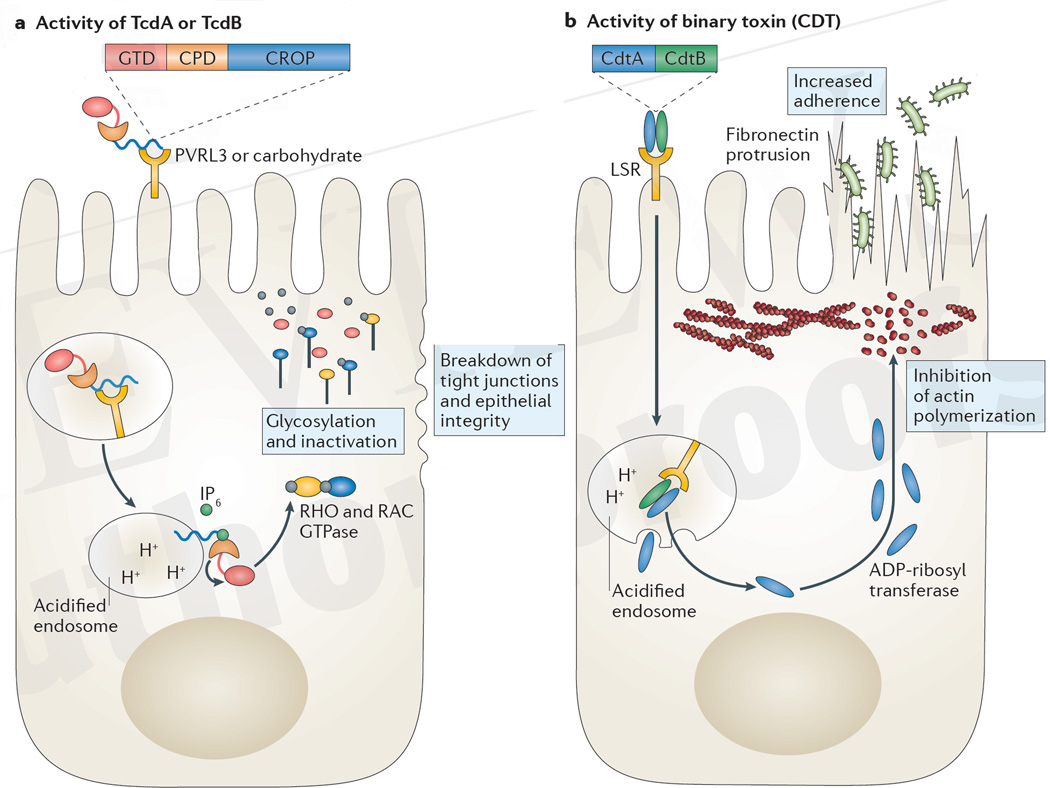
Figure 2. Mechanism of action of C. difficile toxin in epithelial cells.
a | The combined repetitive oligopeptide repeat (CROP) domain of TcdA binds to carbohydrates on the apical surface of epithelial cells, whereas TcdB binds to poliovirus receptor-like 3 (PVRL3) expressed on colonic epithelial cells. Toxin is internalized and acidification of the endosome enables the CROP domain to embed into the endosomal membrane and the subsequent transport of the cysteine protease domain (CPD) and the glucosyl transferase domain (GTD) into the cytosol. Inositol hexakisphosphate (IP6) activates the cysteine protease to cleave and release the toxin glycotransferase. Glycosylation and thereby inactivation of RHO or RAC GTPases ultimately results in the breakdown of tight junctions and epithelial integrity. b | Binary toxin (also known as Clostridium difficile transferase (CDT)) binds to the lipolysis-stimulated lipoprotein receptor (LSR) and is internalized. The CdtB subunit creates pores in the acidified endosome that enable the release of the CdtA subunit into the cytosol. The ADP-ribosyl transferase activity of CdtA inhibits actin polymerization near the cell membrane, enabling fibronectin microtubules to elongate and protrude through microvilli, which increases C. difficile adherence to the epithelium through type IV pili.