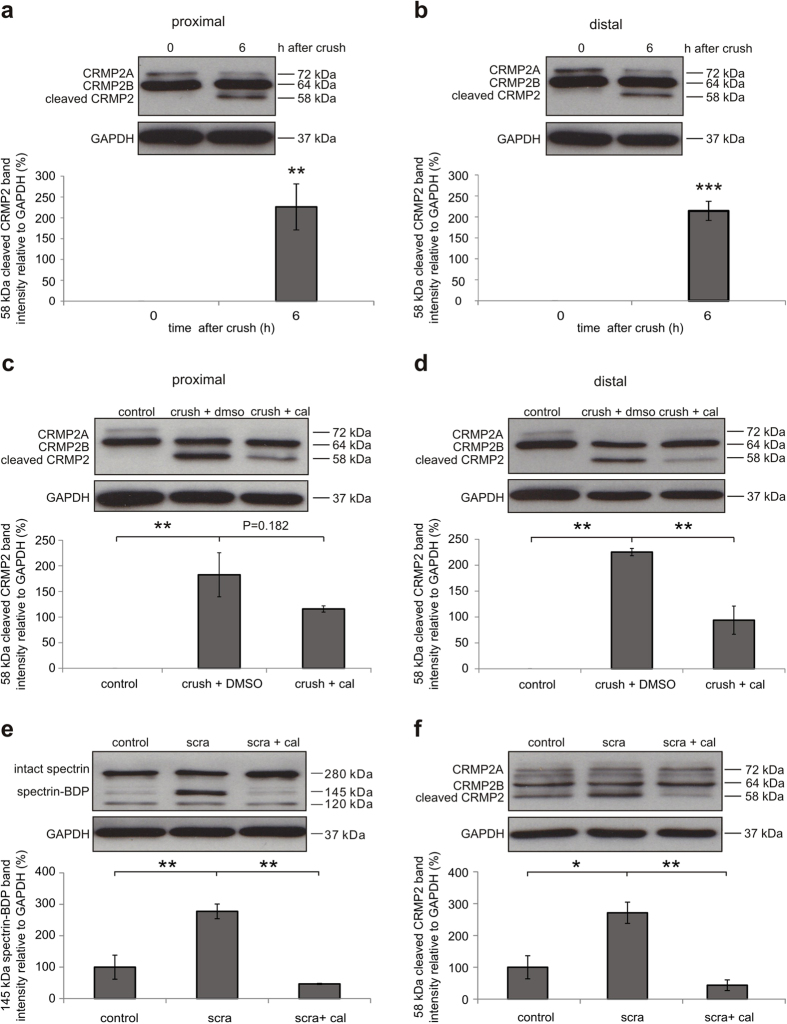
Figure 3. Calpain-mediated cleavage of CRMP2 during acute axonal degeneration in vivo and after scratch in vitro.
(a,b) Representative Western blots of CRMP2 proximal (a) and distal (b) to the crush site in uncrushed native optic nerves (0 h) and in optic nerves at 6 h after crush. Below, quantification of cleaved CRMP2 band intensity relative to GAPDH. 4 optic nerves per group. Error bars represent standard error of the mean (SEM). **P < 0.01, *** P < 0.001 by independent samples t-test. (c,d) Representative Western blots of CRMP2 proximal (c) and distal (d) to the crush site in uncrushed native optic nerves (control), in optic nerves at 6 h after crush pretreated with 7% DMSO (crush + DMSO), and in optic nerves at 6 h after crush pretreated with 10 mM calpeptin in 7% DMSO (crush + cal). Cal = Calpeptin. Quantifications of cleaved CRMP2 band intensity relative to GAPDH are shown below. Four optic nerves are included in each group. Error bars represent the standard error of the mean (SEM). **P < 0.01 by one-way ANOVA and Dunnett’s test. (e,f) Representative Western blots of spectrin (e) and CRMP2 (f) in unscratched primary cortical neuron cultures pretreated with 0.1% DMSO (control), scratched cultures pretreated with 0.1% DMSO (scra + DMSO), and scratched cultures pretreated with calpeptin in 0.1% DMSO (scra + cal). Cal = calpeptin. Sca = scratch. Below, band intensities of 145 kDa cleaved spectrin (e) and cleaved CRMP2 (f) were quantified and normalized to GAPDH. 3 independent cultures per group. Error bars represent the standard error of the mean (SEM). *P < < 0.05, **P < 0.01 by one-way ANOVA and Dunnett’s test.