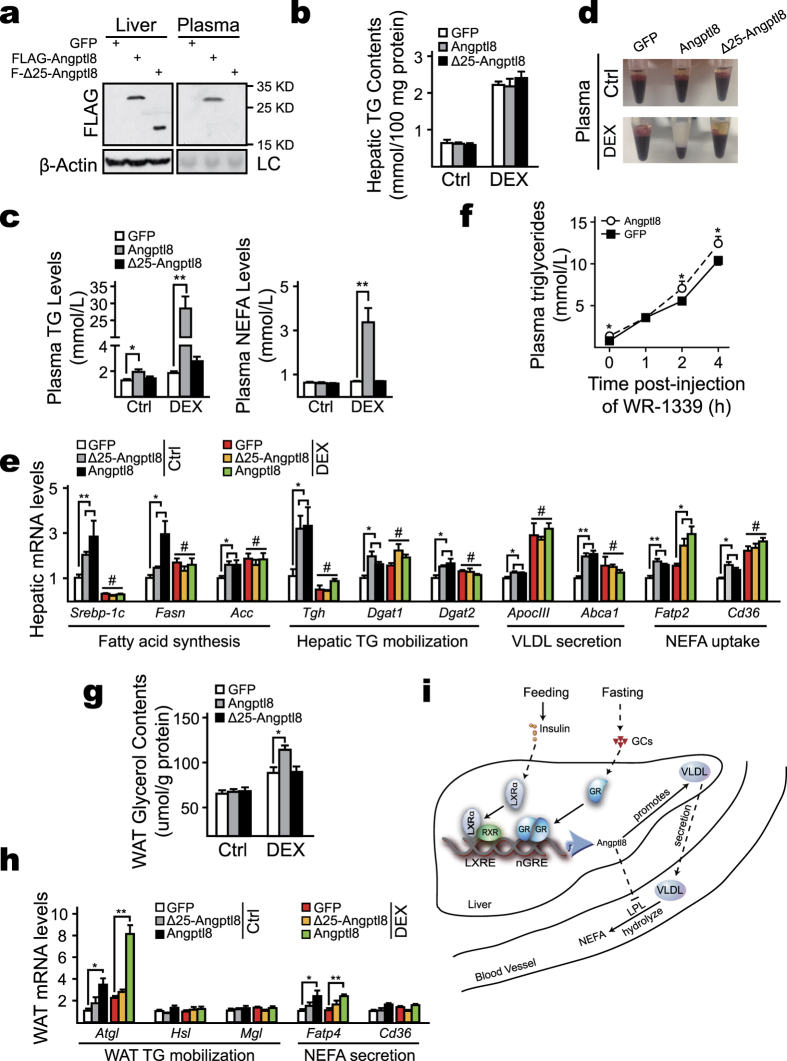
Figure 6. Metabolic interaction between Angptl8 and glucocorticoid signaling.
Ad-GFP, Ad- Angptl8 or Ad-Δ25- Angptl8 adenoviruses were delivered into mice via tail-vein injection on Day1. Mice were treated with vehicle (PBS) or DEX (100 mg/Kg) by intraperitoneal injection once-daily for 3 days from Day4 to Day6, and then animals were euthanized on Day10. (a) Immunoblotting analysis of hepatic and plasma Angptl8 and Δ25- Angptl8. (b) Measurement of hepatic TG contents. (c) Measurement of plasma TG and NEFA levels of indicated virus-infected mice. (d) A picture of plasma shows that ectopic expression of Angptl8 results in cream-like plasma in DEX-treated mice. (e) Quantitative PCR analysis of mRNA levels of hepatic genes involved in lipid metabolism. (f) Plasma TG levels post-injection of Triton WR-1339 (500 mg/Kg) at the indicated time points. (g) Overexpressing Angptl8 promotes lipolysis in adipocytes in DEX-treated mice n = 4–8, *p < 0.05, **p < 0.01, # no significant difference. (h) Overexpressing Angptl8 affects gene expression involved in lipolysis in WAT. (i) Hypothetical model showing the mechanism of fasting and feeding signals modulate Angptl8 expression in mouse liver. Postprandially, the liver X receptor alpha (LXRα) heterodimerizes with retinoid X receptor (RXR) and binds to the liver X receptor response element (LXRE) in the promoter of Angptl8 to initiate its transcription. During fasting state, elevated glucocorticoids suppress Angptl8 transcription by activating glucocorticoid receptor (GR) and subsequent binding of GR dimmers to the negative glucocorticoid response element (nGRE) in the promoter of Angptl8. Elevated Angptl8 increases plasma TG concentration, whereby promoting hepatic VLDL secretion and decreasing VLDL hydrolization via inhibiting LPL activity.