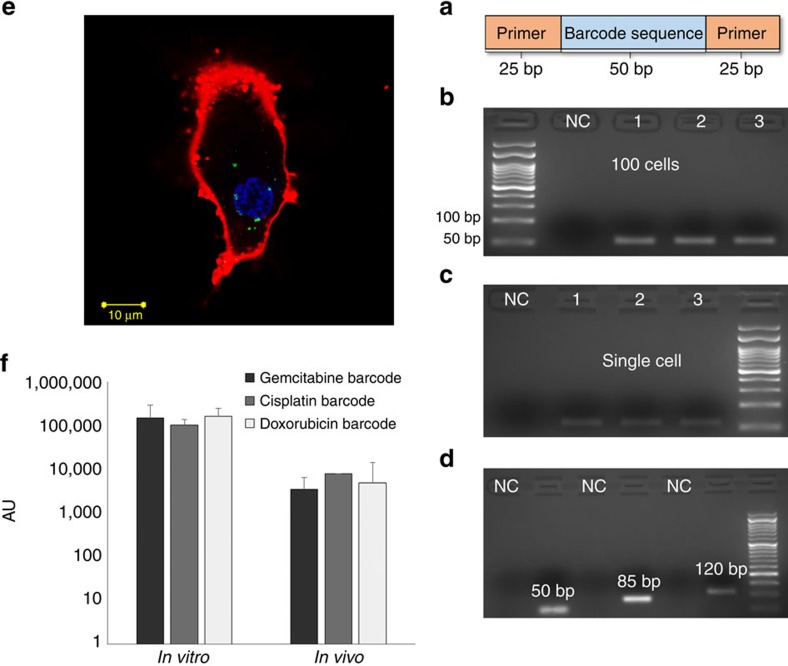
Figure 2. DNA was used as a barcode for labelling and detecting nanoparticles in single cells.
Synthetic DNA strands were embedded in 100 nm liposome together with a corresponding drug. (a) The barcodes were constructed to be in the range of 50 to 120 bp long, and detected using PCR and sequencing. Barcoded nanoparticles were taken up spontaneously by triple-negative breast cancer cells in culture and in tumours. (b) Gel electrophoresis of PCR-amplified barcodes derived from 100 cells, and (c) from a single cell. (d) Different strand lengths of barcodes (50, 85, 120 bp) can be detected within a single-cell suspension. Negative control wells are designated NC, and repeats are numbered 1–3. (e) A confocal microscopy image of uptake of BNPs by a triple-negative breast cancer 4T1 cell labelled for membrane (red), nucleus (blue) and DNA barcodes (green). The single-cell uptake of barcoded nanoparticles is not affected by the cargo (f). Barcoded nanoparticles, all 100 nm in diameter but loaded with different cargo, were added to triple-negative breast cancer cells in culture, or injected intravenously into tumour-bearing mice. To ensure the uptake of multiple particles per cell, a dose 1,000 times higher than that used for the diagnostic procedure was administered. The cells were collected from the dish (after 24 h), or a biopsy was taken from the tumour (after 48 h). The tissue was then dissociated, and 60 individual cells were examined for the presence of each of the different barcodes. Each cell contained a similar number of each of the barcodes, indicating that the internal payload of the nanoparticles does not affect their cellular uptake. The data were calculated as the mean±s.e.m. of n=60.