Abstract
Patients with extremely severe oligozoospermia (ESO) and cryptozoospermia (CO) are suitable using intracytoplasmic sperm injection (ICSI) as an infertility treatment. However, some andrologists are confused to distinguish ESO and CO in clinic diagnose. This study was designed for the first time to evaluate and compare patients with ESO and CO to determine whether these are useful clinical distinctions. A total of 270 infertile men in our center were classified into four groups as Group nonobstruction azoospermia (NOA, n = 44), Group ESO (n = 78), Group CO (n = 40), and Group obstruction azoospermia (OA, n = 108). Comparisons of the volume of bilateral testes, the level of follicle stimulating hormone (FSH) and inhibin B were obtained in four groups. Then comparisons of fertilization rates, cleavage rate, and excellent embryos rate were obtained when couples performed ICSI. All indexes (volume of bilateral testis, level of FSH and inhibin B) in Groups ESO and CO were no difference, while Groups OA versus NOA, OA versus ESO, and OA versus CO were significant differences (P < 0.05). The rates of fertilization were no differences in Groups ESO and CO while Groups OA versus ESO, OA versus CO were significant differences (P < 0.05). Therefore, the spermatogenic functions in patients with CO and ESO were similar, better than NOA but worse than OA. However, it would be helpful to evaluate their spermatogenesis using testicular biopsies, especially accompanied azoospermia in clinical practice.
Keywords: cryptozoospermia, extremely severe oligozoospermia, nonobstruction azoospermia, obstruction azoospermia, spermatogenesis
INTRODUCTION
Cryptozoospermia (CO) is a situation defined by the World Health Organization that spermatozoa cannot be observed in a fresh semen sample. However, it could be found after an extended centrifugation and microscopic search.1 On the other hand, spermatozoa could be found in part of semen samples under microscopic search directly, but could not be counted due to extremely low concentration, and we diagnosed such condition as extremely severe oligozoospermia (ESO). ESO, which has not been defined by the World Health Organization specifically, is a situation that concentration of spermatozoa <1 × 106 ml−1, according to the definition of severe oligoasthenoteratozoospermia by some researchers.2,3 ESO and CO patients are suitable using intracytoplasmic sperm injection (ICSI) as infertility treatment, because their wives are difficult to obtain pregnancy naturally or by drug treatment. ICSI helps them to be biological parents by utilizing their single spermatozoa that have been obtained from ejaculation or percutaneous epididymal sperm aspiration (PESA) or testicular sperm aspiration (TESA), even testicular sperm extraction (TESE) or micro-TESE.
Zoospermia most refers to three sperm parameters such as total sperm concentration, motility, and normal morphology. However, when the concentration of spermatozoa <2 × 106 ml−1, it is hard to observe motility and normal morphology. Both in ESO and CO patients, some andrologists are confused to distinguish the difference between both concepts in clinic diagnose, even consider both concepts are the same meaning. Hence, are ESO and CO the same concept or whether we distinguish them? Our current study was designed for the first time to compare and respond this question.
MATERIALS AND METHODS
Study design
From 2012 to 2014, a total of 270 men were included in the study that Group NOA comprised nonobstructive azoospermia (NOA, n = 44), Group ESO comprised ESO (n = 78), Group CO comprised CO (n = 40), and Group OA comprised obstructive azoospermia (OA, n = 108). All patients were infertile men who referred to the Center for Reproductive Medicine, Nanfang Hospital, Guangzhou, Guangdong, China. Both criteria of NOA and OA were, no sperm in semen after an extended centrifugation, and differentiation between NOA and OA was distinguished by history, physical examination, laboratory investigation, karyotype, Y chromosome microdeletion test, and testis biopsy.4 Inclusion criteria of ESO and CO were sperm concentration <2 × 106 ml−1 of semen directly or after an extended centrifugation (1500 ×g for 15 min), respectively.5 According to the WHO guidelines, semen analysis in whole patients was performed more than 2 times.
All infertility couples in this study had excluded the female factors such as endometrial polypus, myoma, uterus deformation, and endometriosis. All male patients were performed for physical examination, levels of follicle stimulating hormone (FSH) and inhibin B, Y chromosome microdeletions, and standard chromosome analysis. Then, patients who underwent ICSI were compared with the outcome.
Physical examination
The genital examinations include inspections of the body habitus, the external genitalia, and the scrotal contents. The testis size, character and the presence of testicular mass, or asymmetry was assessed via manual palpation and orchidometer.
Hormone analysis
Blood samples were collected and centrifuged after clotting, then stored at −20°C until analysis. Serum inhibin B concentrations were measured using an availably purchased, double antibody enzyme-linked immunosorbent assay kits (Serotec, Oxford, UK). The detection limit was 20 pg·l−3 with a coefficient variation of 12%–17%. Serum FSH concentrations were determined by time-resolved immune fluorometric assays (Delfia, Turku, Finland). The detection range was 1.4–18.1 IU·l−1.
Semen processing
Spermatozoa obtained from ejaculation in Groups ESO and CO or extracted by PESA6 or TESA6 in Group OA were collected. Then, the sperm samples were maintained to liquefy for at least 20 min on a warm plate at 37°C before analysis. Then, sperm precipitate was divided into the injection plate in multiple droplets of culture medium, and a carefully extended sperm examination was made to find viable motile semen for ICSI.
Oocyte recovery
Ovarian stimulation was accomplished using the routine long protocol of luteal suppression using a gonadotropin-releasing hormone agonist. Oocyte recovery was retrieved under vaginal ultrasound guidance. After ICSI procedure, the greatest embryos were selected, and embryo transfer was done on day 3 behind oocyte retrieval.
ICSI procedure
According to conventional operation in our center,6 a viable sperm with normal morphology was selected from washed semen precipitates in culture medium and picked up using an injection pipette filled with a small number of polyvinylpyrrolidone (PVP; Vitrolife, Goteborg, Sweden). A selected sperm was washed into the PVP micro-droplet to remove surrounding debris particles that could be harmful for oocyte and resulted embryo. After injection, the oocytes were irrigated and incubated in G-IVF medium (Vitrolife, Goteborg, Sweden). ICSI procedure was performed at 37°C under a warmed-stage inverted microscope at ×200 magnification.
Outcomes
Fertilization of pronucleus stage (2PN) was assessed at 16–18 h after microinjection. On day 3 behind fertilization, the grade of embryos was evaluated according to morphology, fragmentation, and blastomeres. Excellent embryos were no more than 10% fragmentation and 7–8 symmetric and regular blastomeres.
Statistical analysis
Calculations were analyzed using SPSS 19.0 software (SPSS Inc., Chicago, Illinois, USA). All numeric data were presented as the mean value ± standard deviation. Frequencies were expressed as percentages. The statistical analysis was performed using unpaired t-test between two groups, whereas χ2 test was used for comparison of proportions. Comparison of mean values among more than three groups was performed using analysis of variance test. Differences between the values were considered statistically significant when P < 0.05.
RESULTS
Characteristics of patients
As shown in Table 1, there was no difference in age of male in four groups (P = 0.683). Some spermatogenic function parameters (volume of bilateral testes, level of FSH and inhibin B) are summarized in Table 1 and significant differences in four groups (P = 0.000, P = 0.000, P = 0.000, respectively). Then, comparisons between groups are shown in Figure 1.
Table 1.
Characteristics of patients
Figure 1.
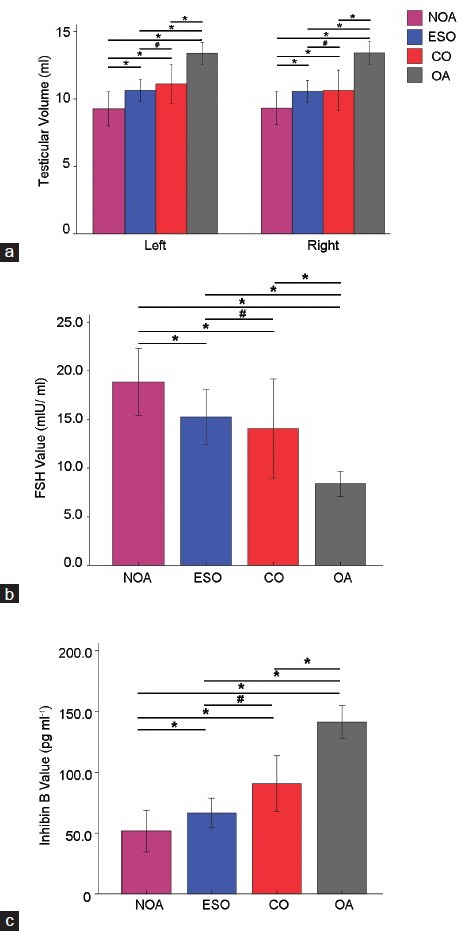
Comparisons among four groups of volume of bilateral testis (a), the level of FSH (b) and inhibin B (c). NOA: nonobstructive azoospermia; ESO: extremely severe oligozoospermia; CO: cryptozoospermia; OA: obstructive azoospermia; *P < 0.05; #P > 0.05.
The volume of bilateral testes was biggest in Group OA, moderate in Groups ESO and CO, and smallest in Group NOA. There was no difference in Groups ESO and CO while Groups OA versus NOA, OA versus ESO, and OA versus CO were significant differences (P = 0.000, P = 0.000, P = 0.005, respectively).
FSH was remarkably elevated in Group NOA, moderate in Groups ESO and CO, and lowest in Group OA. FSH in Group ESO (15.24 ± 12.60 IU·l−1) and Group CO (14.07 ± 14.11 IU·l−1) was no effective difference, but it reached statistical significance with Group NOA (18.88 ± 10.62 IU·l−1) and Group OA (8.39 ± 6.89 IU·l−1).
Concordantly, in the same group of patients, inhibin B tended to be opposite to FSH. Similarly, inhibin B in Group ESO (66.59 ± 54.48 pg·l−3) and Group CO (90.76 ± 63.55 pg·l−3) was no effective difference, but it reached statistical significance with Group NOA (51.69 ± 52.98 pg·l−3) and Group OA (141.53 ± 71.82 pg·l−3).
Outcomes of ICSI
As shown in Table 2, the age of female was comparable as there were no statistical differences in three groups (P > 0.05). Outcomes of ICSI (ratio of fertilization, oocyte cleavage, and excellent embryos) are summarized in Table 2 and Figure 2.
Table 2.
Outcomes of ICSI
Figure 2.
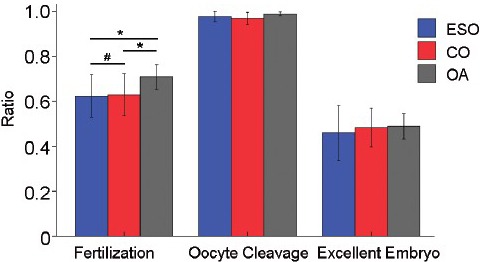
Comparisons among three groups of rates of fertilization, oocyte cleavage, and excellent embryos. ESO: extremely severe oligozoospermia; CO: cryptozoospermia; OA: obstructive azoospermia; *P < 0.05; #P > 0.05.
Fertilization rates increased in Group OA and were remarkable differences in three groups (P = 0.049). Then, comparisons between groups were performed. There was no difference in Groups ESO and CO (64.95% vs 64.52%, P = 0.928), but Groups OA versus ESO, OA versus CO were significant differences (P = 0.049, P = 0.047, respectively).
However, oocyte cleavage rates were no statistical significance in three groups (P = 0.313), and excellent embryos rates were the same (P = 0.576).
DISCUSSION
Over the past several decades, in vitro fertilization and ICSI have come into routine practice. However, studies in male infertility were much less than in female infertility although ICSI is mainly used for severe male factors such as OA, CO, and ESO. Some basic definitions and concepts such as CO and ESO were not clarified by most doctors and researchers. In many situations, we often confused the concept of CO and ESO. According to the WHO laboratory manual,1 CO is defined that spermatozoa absent from fresh preparations but observed in a centrifuged pellet. It is affected by climate temperature, the environment of masturbation room, the mood in ejaculation, stimulation by audio-visual equipment and others, and sperm count in CO changed remarkably. Because, sometimes CO was accompanied with azoospermia in part of patients, some author classified CO under the NOA category.7 However, scarce sperms could be found fitfully in some ejaculation; hence, it is unsuitable to included CO under NOA category.
To the best of our knowledge, this is the first study to emphasize the concept of CO and ESO. ESO has not been defined by the WHO, and the number of spermatozoa was not unified and different in research. Most authors such as Lu et al.2 defined number of severe oligoasthenoteratozoospermia <1 × 106 ml−1, but Koscinski et al.3 defined a number of extremely oligozoospermia <1 × 103 ml−1. Similar with CO, sperm count in ESO also changed remarkably depended on many elements as previously mentioned. In our opinions, ESO refers to one or a few of sperms can be found in one or several high power field. While the number of spermatozoa <2 × 106 ml−1, it is difficult to give a specific value in our lab, hence we recommended that criteria of ESO were sperm concentration <2 × 106 ml−1.
ICSI was a revolutionized technique that was able to bypass barriers in natural sperm and oocyte interaction and fusion, especially suitable for serious oligozoospemia such as CO and ESO. However, no sperm is found on the day of oocyte retrieval that leads to the cancelation of ICSI only. Although testis biopsy is the gold standard in evaluating spermatogenesis, it is not a regular examination in CO and ESO. Therefore, it is necessary to evaluate such patients’ spermatogenic functions via volume of testis size, levels of FSH and inhibin B in advance. Whereas, we also recommend that testicular biopsy need to be performed while CO and ESO accompanied with azoospermia.
A lot of comparative studies considered that relative testis size and sperm production were strongly correlated, and testis size would be a useful means of evaluating a male investment in sperm production.8 Compared to European and American people, testicular volumes of 13 ml in OA seemed to be atrophic in our study, but the testicular volumes of normal men also were 13 ml averagely (data not showed). According to the volume of bilateral testes in our study, our preliminary thought for spermatogenic functions in CO and ESO were similar, better than NOA but worse than OA. However, focusing solely on this examination but ignoring other examination of testicular function might ultimately impede our attempts to understand the function and evolution of this important organ.
FSH has considerable biological roles in testicular function, such as cellular sperm differentiation, maturation of epididymal sperm, and adjustment of spermatid morphogenesis.9 Lacking of FSH would shorten testis size, sperm concentration, and sperm motility, hence FSH plays an important role in boosting cells proliferation and is desired for normal sperm production.10,11 Similar to testicular volumes, FSH level of 8.39 IU·L−1 seems to be slightly high compared to other research,12 but FSH level was 1.4–18.1 IU·l−1 in our clinical lab. In our study, we found that FSH was elevated in Group NOA, moderate in Groups ESO and CO (no significant difference), and lowest in Group OA. Such results also support that spermatogenic functions in CO and ESO were similar, better than NOA but worse than OA.
Inhibin B, which is likely to be the crucial feedback regulator of FSH secretion,13 is associated well with FSH in sperm concentration, and thus support them as serum characters of spermatogenesis.14 While inhibin B is maintained in detectable serum levels, spermatogenic activity in adults is required.15 The inhibin B levels were dramatically lower in male with a spermatogenic defect. In our study, we found that inhibin B tended to be opposite to FSH in the same group. Therefore, FSH and inhibin B concentrations conjointly reflected the capacity of spermatogenesis in CO and ESO were similar, better than NOA but worse than OA.
Although spermatogenic function index (volume of testis size, levels of FSH and inhibin B) of these patients were in normal reference value range, spermatogenic activities were different from NOA and OA. There were significant differences in these indexes in Groups OA versus CO, OA versus ESO, NOA versus CO, and NOA versus ESO. Such results illustrated that the spermatogenic function of ESO and CO was similar, better than NOA but worse than OA. However, spermatogenic functions of ESO and CO could be directly correlated to the testicular status based on testicular biopsy, and it would be helpful to evaluate their spermatogenesis by testicular biopsies.
After completing ICSI, all parameters were analyzed except Group NOA, because these patients were so difficult to obtain sperm that only using artificial insemination by donor. Fertilization rates were no difference in Groups ESO and CO but the significant difference among other groups’ comparisons (OA vs. ESO, OA vs. CO). In order to a standard rate of oocyte cleavage and excellent embryonic development, we chose sperm at the margin of the PVP micro-droplet using ×400 magnification to ensure that the selected semen had considerably normal form and fine motility. Fertilization rates further illustrated that the capacity of spermatogenesis in ESO and CO was similar but worse than OA.
However, ESO and CO do not represent etiologies, but just interpretation of semen parameters. Consequently, some patients may have testicular dysfunction such as microdeletion of Y chromosome, and others may have partial seminal tract obstruction in this cohort while their spermatogenesis is normal as OA. This can cause heterogeneity of patients in ESO and CO Groups. The key point in this study is to clarify the difference between these two concepts. Also, the number of cases was not big enough and it was not suitable to compare subtype due to testicular dysfunction.
CONCLUSION
The spermatogenesis in patients with CO and ESO were similar, better than NOA but worse than OA. However, the number of sperms in CO and ESO was different in laboratory detection. Moreover, both CO and ESO were a description of the ejaculate, rather than diagnoses. Thus, it would be helpful to evaluate their spermatogenesis by testicular biopsies, especially accompanied azoospermia in clinical practice.
AUTHOR CONTRIBUTIONS
YTZ conceived of this study, collected data, performed data analysis, and prepared the manuscript. CL collected data, performed data analysis, and prepared the manuscript. YL collected data. HL collected data. SQ collected data. YJD performed data analysis. YY collected data. WLT conceived of this study and prepared the manuscript. QJC conceived of this study, performed data analysis, and prepared the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declared that they have no competing interests.
ACKNOWLEDGMENTS
This research was supported by the Scientific Initiative Research Foundation of Southern Medical University (No. PY2014N031).
REFERENCES
- 1.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.Lu YH, Gao HJ, Li BJ, Zheng YM, Ye YH, et al. Different sperm sources and parameters can influence intracytoplasmic sperm injection outcomes before embryo implantation. J Zhejiang Univ Sci B. 2012;13:1–10. doi: 10.1631/jzus.B1100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koscinski I, Wittemer C, Lefebvre-Khalil V, Marcelli F, Defossez A, et al. Optimal management of extreme oligozoospermia by an appropriate cryopreservation programme. Hum Reprod. 2007;22:2679–84. doi: 10.1093/humrep/dem190. [DOI] [PubMed] [Google Scholar]
- 4.Wosnitzer M, Goldstein M, Hardy MP. Review of Azoospermia. Spermatogenesis. 2014;4:e28218. doi: 10.4161/spmg.28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corea M, Campagnone J, Sigman M. The diagnosis of azoospermia depends on the force of centrifugation. Fertil Steril. 2005;83:920–2. doi: 10.1016/j.fertnstert.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Qiu ZL, Chu QJ, Mao XM, Luo C, Quan S. [Embryo development potential after intracytoplasmic injection of sperm from azoospermia patients with different spermatogenic functions] Zhonghua Nan Ke Xue. 2012;18:432–5. [PubMed] [Google Scholar]
- 7.Bendikson KA, Neri QV, Takeuchi T, Toschi M, Schlegel PN, et al. The outcome of intracytoplasmic sperm injection using occasional spermatozoa in the ejaculate of men with spermatogenic failure. J Urol. 2008;180:1060–4. doi: 10.1016/j.juro.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Ramm SA, Scharer L. The evolutionary ecology of testicular function: size isn’t everything. Biol Rev Camb Philos Soc. 2014;89:874–88. doi: 10.1111/brv.12084. [DOI] [PubMed] [Google Scholar]
- 9.Condorelli RA, Calogero AE, Vicari E, Mongioi’ L, Burgio G, et al. Reduced seminal concentration of CD45pos cells after follicle-stimulating hormone treatment in selected patients with idiopathic oligoasthenoteratozoospermia. Int J Endocrinol 2014. 2014 doi: 10.1155/2014/372060. 372060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120:956–62. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarulli GA, Stanton PG, Meachem SJ. Is the adult Sertoli cell terminally differentiated? Biol Reprod. 2012;87:13, 1–11. doi: 10.1095/biolreprod.111.095091. [DOI] [PubMed] [Google Scholar]
- 12.Eckardstein S, Simoni M, Bergmann M, Weinbauer GF, Gassner P, et al. Serum inhibin B in combination with serum follicle-stimulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab. 1999;84:2496–501. doi: 10.1210/jcem.84.7.5855. [DOI] [PubMed] [Google Scholar]
- 13.Pierik FH, Vreeburg JT, Stijnen T, De Jong FH, Weber RF. Serum inhibin B as a marker of spermatogenesis. J Clin Endocrinol Metab. 1998;83:3110–4. doi: 10.1210/jcem.83.9.5121. [DOI] [PubMed] [Google Scholar]
- 14.Andersson AM, Petersen JH, Jorgensen N, Jensen TK, Skakkebaek NE. Serum inhibin B and follicle-stimulating hormone levels as tools in the evaluation of infertile men: significance of adequate reference values from proven fertile men. J Clin Endocrinol Metab. 2004;89:2873–9. doi: 10.1210/jc.2003-032148. [DOI] [PubMed] [Google Scholar]
- 15.Petersen PM, Andersson AM, Rorth M, Daugaard G, Skakkebaek NE. Undetectable inhibin B serum levels in men after testicular irradiation. J Clin Endocrinol Metab. 1999;84:213–5. doi: 10.1210/jcem.84.1.5406. [DOI] [PubMed] [Google Scholar]


