Abstract
The corticostriatothalamic circuit regulates learning behaviors via dopamine neurotransmission. D2 long (D2L) receptors are an isoform of dopamine D2 receptors (D2Rs) and may act mainly at postsynaptic sites. It is well known that D2Rs influence high brain functions, but the roles of individual D2R isoforms are still unclear. To assess the influence of D2L receptors in visual discrimination learning, we performed visual discrimination and reversal tasks with D2L knockout mice using a touchscreen operant system. There were no significant differences in an operant conditioning task between genotypes. However, D2L knockout mice were impaired in both visual discrimination and reversal learning tasks. D2L knockout mice were also significantly slower than wild-type mice in collecting the reward in the visual discrimination task. These results indicate that D2L receptors play an important role in visual discrimination and reversal learning.
Key Words: Dopamine receptor, Touchscreen, Flexibility
Introduction
Visual discrimination learning requires perceptual learning and memory processing [1,2], while reversal learning assesses behavioral flexibility of learning [3,4]. Abnormalities in visual discrimination learning have been shown in patients with depression, autism, and Alzheimer's disease, while impairments of reversal learning have been shown in patients with schizophrenia and Alzheimer's disease [5,6,7,8,9]. The corticostriatothalamic circuitry connecting the prefrontal cortex, striatum, and thalamus is important for learning behaviors in humans, nonhuman primates, and rodents [10,11,12]. Dopamine transmission is a major modulator of this circuit [11,13,14].
Dopamine receptors are classified into two subfamilies, namely D1-like (D1 and D5) and D2-like (D2, D3, and D4) [15]. D2 receptors (D2Rs) have two alternative splicing variants: long-form (D2L) receptors and short-form (D2S) receptors. D2L receptors may act predominantly at postsynaptic sites, whereas D2S receptors may act predominantly at presynaptic sites [16,17,18]. Changes in expression levels of each isoform have been reported for the dorsolateral prefrontal cortex and hippocampus in schizophrenia and affective disorders [19]. Mice lacking presynaptic D2 autoreceptors display hyperlocomotion and enhanced motivation [20]. In contrast, locomotor activity and the reinforcing properties of food rewards are reduced in D2R-deficient mice lacking both the D2L and the D2S isoform [21,22,23]. Similarly, D2L receptor-deficient mice also show hypolocomotion and reduced motivation [24,25,26]. These findings demonstrate that presynaptic D2S and postsynaptic D2L receptors play distinct roles in motor and motivational functions. However, it is still unclear whether D2L and D2S receptors play individual roles in controlling visual discrimination and reversal learning.
In recent years, the development of touchscreen operant chambers has allowed researchers to assess high brain functions in humans, nonhuman primates, and rodents with a high degree of automation and standardization [27,28,29]. Many translatable tests, such as visual discrimination (VD) and reversal learning (VDR) tasks, using touchscreen operant systems have been developed [30]. Visual discrimination and reversal learning, measured using touchscreen operant systems, have been reported in disease model mice [31,32,33,34,35]. GluA1 mutant mice, used as a model of schizophrenia, show impairments of reversal learning [33], while 16p11.2 mutant mice, used as a model of autism, show impairments of visual discrimination learning [35].
Here we used a gene knockout mouse model to investigate the role of D2L receptors in the performance of touchscreen tasks. We showed that D2L receptor-deficient mice (D2L-KO mice) [24] exhibited impairments in both VD and VDR tasks. D2L-KO mice were also significantly delayed in collecting the reward in the VD task. These findings suggest an important role for D2L receptors in the modulation of visual discrimination learning.
Materials and Methods
Animals
Heterozygous D2L-Het mice [24] were backcrossed to a C57BL/6J genetic background for more than 10 generations; homozygous D2L-KO, heterozygous D2L-Het, and wild-type (WT) littermate mice were generated by mating heterozygous D2L-Het mice. The mice were group housed on a 12-hour light/dark cycle, and all experiments were conducted during the light phase of the cycle. All experiments used 15-week-old male mice. All animal handling procedures were approved by the animal research committees of Kyoto University Graduate School of Medicine.
VD Tasks in the Touchscreen Operant System
Apparatus
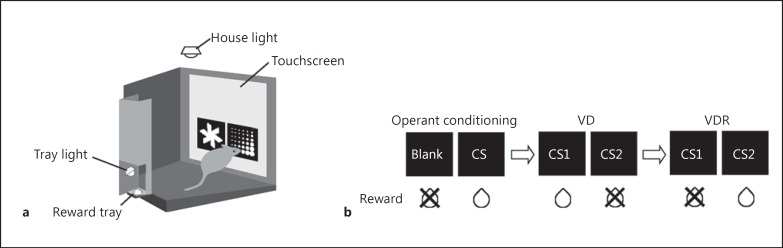
Training and testing were conducted in touchscreen operant chambers (Campden Instruments Ltd., Loughborough, UK) (fig. 1a). The front wall of the chamber consisted of an infrared touchscreen. A black mask with two windows was positioned in front of the touchscreen. The rear wall consisted of a centrally mounted liquid dipper and reward tray, which provided access to 10% condensed milk (Morinaga Milk Industry Co. Ltd., Tokyo, Japan) as a reward, and a tray light located above the reward tray. A house light was mounted above the chamber. The operant conditioning chamber was controlled using ABET II (Lafayette Instrument Co., Lafayette, Ind., USA) and Whisker (Cambridge University Technical Services Ltd., Cambridge, UK) software.
Fig. 1.
a Touchscreen operant chamber. Condensed milk as a reward was dropped from the liquid dipper to the reward tray at the rear wall. b Schematic representation of visual stimuli and reward patterns during each stage of the experiment. Following habituation and pretrainings, the mice performed the operant conditioning, VD, and VDR tasks in sequence. The images below the stimuli indicate the outcome (reward vs. no reward) of touch responses to either CS. In the operant conditioning task, the CS was randomly presented on one side of the screen, and the other side of the screen was left blank. In the VD and VDR tasks, a pair of visual discriminative stimuli (CS1 and CS2) was presented on the screen. The left-right arrangement of the stimuli was determined pseudorandomly.
Pretraining and Operant Conditioning Task
Behavioral protocols and schedules were based on established protocols [30]. Following commencement of testing, the mice were restricted to 4 h of drinking water per day. They were required to successfully fulfill a set criterion for each task before advancing to the next task. First, the mice were habituated to the apparatus by being placed in the chamber for 40 min on 2 consecutive days and exposed to 150 μl of condensed milk in the reward tray.
In the first pretraining schedule, during each trial, visual stimuli were displayed randomly as conditioned stimuli (CS) on one side of the screen, while the other side of the screen was left blank. Various shapes were used for the CS. One stimulus was presented at a time. After a delay (30 s), regardless of any murine responses, the stimulus was removed and 20 μl of condensed milk was delivered as a reward. Reward delivery was accompanied by illumination of the tray light and a tone (3 kHz). When the mouse entered the reward tray to collect a reward, the tray light was switched off and an intertrial interval (ITI) was initiated. After the ITI period (20 s), the tray light was again illuminated. Subsequently, the next trial was initiated by an entry into the reward tray. The session ended following the completion of 30 trials, or after 60 min, whichever came first. The mice progressed to the next training stage following the completion of 30 trials in 60 min.
In the second pretraining schedule, stimuli were again displayed randomly on one side of the screen during a trial. The mice had to touch the stimulus to elicit the tone and reward response. If the mice touched the blank screen, no reward was given. Reward delivery was accompanied by illumination of the tray light and the tone. When the mice entered the reward tray to collect a reward, the tray light was switched off and the ITI was initiated. After the ITI, the tray light was again illuminated. A new trial was initiated by an entry into the food tray. As before, the session ended following the completion of 30 trials, or after 60 min, and the mice progressed to the next stage if they successfully completed 30 trials in 60 min.
The operant conditioning task was conducted similarly to the pretraining schedules described above, except that if a mouse touched the side of the screen opposite the stimulus (blank side) in a trial, the house light was illuminated for a time-out period (5 s) and no reward was given (fig. 1b). The session ended following the completion of 30 trials, or after 60 min, whichever came first. The criterion was set at 23/30 (76.7%) correct trials in 60 min. Regardless of whether this criterion was reached, at least 4 operant conditioning sessions were performed.
VD and VDR Tasks
In the VD task, a pair of novel visual discriminative stimuli (CS1 and CS2) appeared on the screen during each trial; CS1 as a correct stimulus and CS2 as an incorrect stimulus (fig. 1b). A nose poke to the correct stimulus resulted in the tone, tray light, and reward (20 μl of condensed milk); a nose poke to the incorrect stimulus resulted in illumination of the house light, no reward, and a time-out, followed by a correction trial. Correction trials were repeated until the correct stimulus was chosen. The left-right arrangement of the stimuli was determined pseudorandomly, with a constraint that a given stimulus could not appear on the same side of the screen for more than 3 consecutive trials. When the mouse entered the reward tray to collect a reward, the tray light was switched off and the ITI was initiated. After the ITI, the tray light was again illuminated. The next trial was initiated by an entry into the reward tray. The session ended following the completion of 30 trials, or after 60 min, whichever came first. The criterion was set at 24/30 (80%, excluding correction trials) correct trials in 60 min. Regardless of whether this criterion was reached, at least 5 VD task sessions were performed.
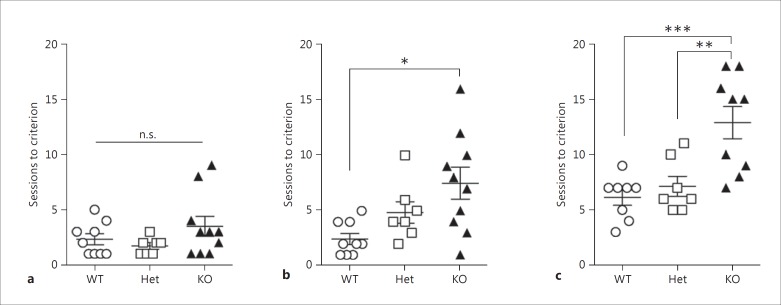
The day after having reached the criterion, the mice were moved on to the VDR task, which followed the same setup as the VD task, except that the designation of the correct versus incorrect stimuli was reversed (fig. 1b). During the VDR task, access to drinking water was further restricted from 4 h per day to 2 h per day to counteract a decrease in motivation likely due to the increased difficulty of the task. The criterion was again set at 80% (excluding correction trials) correct in 60 min. Regardless of whether this criterion was reached, at least 10 VDR task sessions were performed. After 10 sessions in the VDR tasks, 1 of the WT and 1 of the KO mice were lost to accidental death before they reached the criterion; thus these data are missing in figure 3c (see also online suppl. fig. 1c; for all online suppl. material, see www.karger.com/doi/10.1159/000447970).
Fig. 3.
Numbers of sessions to reach the criterion in the operant conditioning (a), VD (b), and VDR (c) tasks. Marks represent the number of sessions to reach the criterion for each mouse. Lines and error bars represent the mean ± SEM. n.s. = Not significant. a, b WT mice: n = 9; D2L-Het mice: n = 7; D2L-KO mice: n = 10. c WT mice: n = 8; D2L-Het mice: n = 7; D2L-KO mice: n = 9. Significance was evaluated by post hoc Dunn's corrections (a, b; n.s., * p < 0.05) and Bonferroni corrections (c; ** p < 0.01, *** p < 0.001).
Statistical and Data Analysis
All statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, Calif., USA) and SPSS 22.0 (IBM Corporation, Armonk, N.Y., USA) software.
Differences in the number of sessions to reach the criterion between genotypes were evaluated using one-way ANOVA or Kruskal-Wallis tests after checking the homogeneity of variance assumption by Bartlett's test [36]. Subsequent post hoc comparisons of genotypic groups were conducted with Bonferroni correction, multiplying pairwise t test p values by the number of tests, or Dunn's correction. Statistical significance was set at p < 0.05.
Differences in the percent of correct responses, correct response latency, and reward collection latency between genotypes were evaluated using two-way, one-repeated-measure ANOVA. Greenhouse-Geisser-corrected degrees of freedom and p values were used [37] whenever Mauchly's test of sphericity was significant [38]. Subsequent post hoc comparisons for the main effect of genotype were conducted with Bonferroni correction. When examining the simple main effect of genotype in each session, the significance level was set at p < 0.05 divided by the number of sessions in order to correct for multiple testing across sessions.
Results
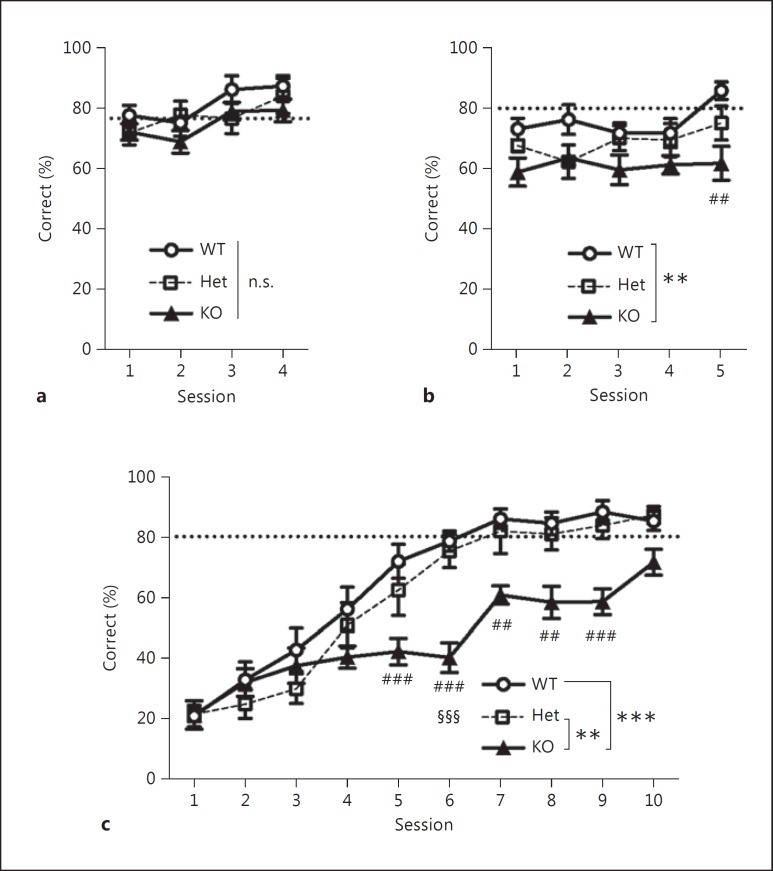
To investigate the role of D2L receptors in learning, we examined the reward-based learning ability of D2L-KO, D2L-Het, and WT mice in VD and VDR tasks using the touchscreen operant systems. After the habituation and pretrainings, the mice performed the operant conditioning task, the VD task, and the VDR task in sequence. In the operant conditioning task, the mice could obtain a reward when they touched a visual stimulus (fig. 1b). WT, D2L-Het, and D2L-KO mice did not differ significantly in their ability to learn the rule following repeated training (fig. 2a). After the mice reached the criterion, they moved on to the VD task (fig. 1b). In this task, the mice could obtain a reward when they touched a correct stimulus from a pair of discriminative stimuli. D2L-KO mice showed fewer correct choices than WT mice in the first 5 sessions of the VD task (fig. 2b). After the mice reached the criterion of 80% correct choices in the VD task, they moved to the VDR task, where the designation of the same discriminative stimuli as correct versus incorrect was reversed (fig. 1b). In the VDR task, WT and D2L-Het mice showed a comparable ability to learn the reversal, and they reached the criterion of 80% correct choices by session 7. D2L-KO mice, however, were impaired in reversal learning and failed to reach the criterion by session 10 (fig. 2c).
Fig. 2.
Percentage of correct responses across sessions in the operant conditioning (a), VD (b), and VDR (c) tasks. Marks and error bars represent the mean ± SEM. WT mice: n = 9; D2L-Het mice: n = 7; D2L-KO mice: n = 10. n.s. = Not significant. The criterion is shown as a dotted line (operant conditioning task: 76.7%; VD and VDR tasks: 80%). Two-way, one-repeated-measure ANOVA showed no significant effects of genotypes and the interaction in the operant conditioning task (a; effect of interaction: F6, 69 = 0.745, p = 0.615; effect of genotypes: F2, 23 = 1.599, p = 0.224), significant effects of genotypes but not the interaction in the VD task (b; effect of interaction: F8, 92 = 1.103, p = 0.369; effect of genotypes: F2, 23 = 7.05, p = 0.004), and significant effects of genotypes and the interaction in the VDR task (c; effect of interaction: F8.827, 101.516 = 3.623, p < 0.001; effect of genotypes: F2, 23 = 13.288, p < 0.001). Post hoc Bonferroni corrections of the overall comparisons between genotypes were performed (** p < 0.01, *** p < 0.001). The comparisons for each session between genotypes were also performed with Bonferroni correction for the number of sessions (b, KO vs. WT: ## p < 0.01/5; c, KO vs. WT: ## p < 0.01/10, ### p < 0.001/10; KO vs. Het: §§§ p < 0.001/10). See online supplementary table 1 for detailed statistical information on the two-way, one-repeated-measure ANOVA.
The acquisition curves of the individual mice are shown in online supplementary figure 1. When two-way, one-repeated-measure ANOVAs were performed for figure 2, the homogeneity of variance was checked with Mauchly's test of sphericity (online suppl. table 1). There were no significant differences in Mauchly's test of sphericity values in figure 2a and b; thus we evaluated p values without corrections by degrees of freedom. On the other hand, there was a significant difference in Mauchly's test of sphericity values in figure 2c, which is why the p values were corrected by the Greenhouse-Geisser method (ε = 0.490). Post hoc Bonferroni corrections were performed in figure 2. In the VD and VDR tasks, we also performed correction trials to counteract side and stimulus biases, and to ensure that the mice received a consistent number of rewards per session despite their performance on noncorrection trials. Regarding the number of correction trials, there were significant effects of genotypes in the VD task (online suppl. fig. 2a) and the interaction between sessions and genotypes in the VDR task (online suppl. fig. 2b). These results correlate with the percent correct responses of D2L-KO mice in the VD (fig. 2b) and the VDR task (fig. 2c). Taken together, these results reveal impairments of discrimination learning in D2L-KO mice in both the VD and the VDR task.
To assess learning ability and flexibility in the VD and VDR tasks, we next measured the number of sessions to reach the criterion in each task. To determine significance between genotypes, we used Bartlett's test to check the homogeneity of variance. There were significant differences in Bartlett's test values in figure 3a and b (fig. 3a, p = 0.0072; fig. 3b, p = 0.015); thus we performed a Kruskal-Wallis test and post hoc Dunn's correction. On the other hand, there was no significant difference in Bartlett's test values in figure 3c (p = 0.0855), which is why we performed one-way ANOVA and post hoc Bonferroni correction. There were no significant differences between genotypes in the time taken to reach the criterion in the operant conditioning task (fig. 3a, p = 0.341), although some D2L-KO mice required more sessions than did WT and D2L-Het mice. D2L-KO mice required more sessions to reach the criteria in the VD (fig. 3b, p = 0.013) and VDR (fig. 3c, F2, 21 = 11.07, p < 0.001) tasks.
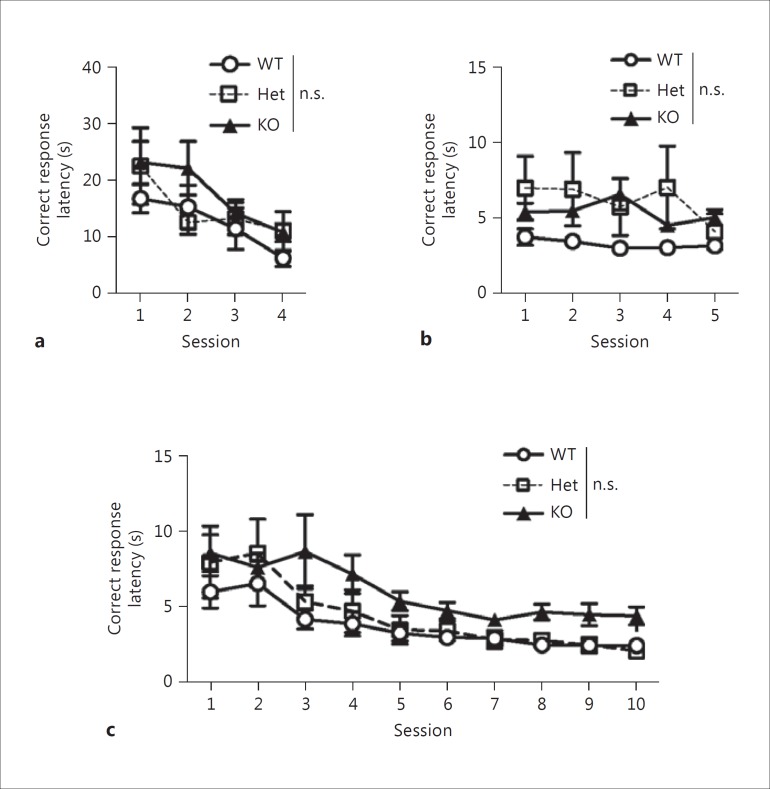
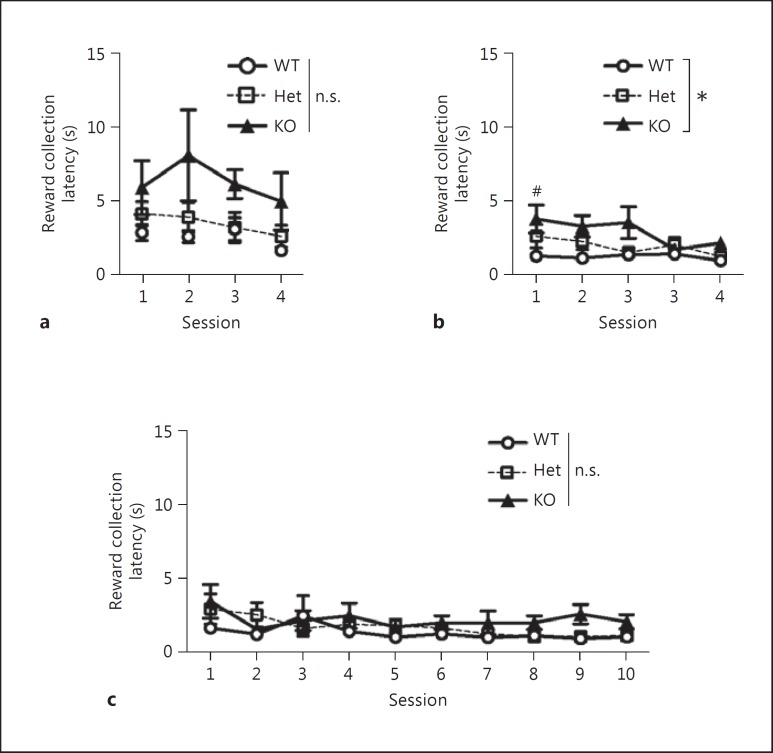
Previously, D2L-KO mice have been shown to display behavioral changes in locomotion [24,25], emotion [39,40], motivation [26,41], and avoidance [25,42,43]. These behavioral changes may influence visual discrimination and reversal learning ability. To address this possibility, we assessed the latency to respond to the correct stimulus and to collect a reward. When two-way, one-repeated-measure ANOVAs were performed on figures 4 and 5, the homogeneity of variance was checked with Mauchly's test of sphericity (online suppl. table 1). There were significant differences in Mauchly's test of sphericity values for figures 4 and 5; thus the p values were corrected using the Greenhouse-Geisser method (fig. 4a, ε = 0.673; fig. 4b, ε = 0.553; fig. 4c, ε = 0.335; fig. 5a, ε = 0.697; fig. 5b, ε = 0.693; fig. 5c, ε = 0.513). Post hoc Bonferroni corrections were performed on figures 4 and 5. Latencies to correct response did not differ significantly between genotypes in each of the tasks (fig. 4), indicating that D2L-KO mice displayed normal response speeds to the correct stimulus. However, D2L-KO mice were significantly slower than WT mice in collecting the reward in the VD task (fig. 5b), indicating that D2L-KO mice may have reduced motivation or reward sensitivity. There were no significant differences between genotypes in the VDR task (fig. 5c), indicating that the low percentage of correct responses and the higher number of sessions to reach the criterion seen in D2L-KO mice reflects a low discriminative ability but not altered motivation.
Fig. 4.
Correct response latencies in the operant conditioning (a), VD (b), and VDR (c) tasks. Marks and error bars represent the mean ± SEM. WT mice: n = 9; D2L-Het mice: n = 7; D2L-KO mice: n = 10. n.s. = Not significant. Two-way, one-repeated-measure ANOVA showed no significant effects of genotypes and the interaction in the operant conditioning (a; effect of interaction: F4.041, 46.471 = 1.124, p = 0.357; effect of genotypes: F2, 23 = 0.895, p = 0.422), VD (b; effect of interaction: F4.428, 50.917 = 1.477, p = 0.219; effect of genotypes: F2, 23 = 2.625, p = 0.094), and VDR (c; effect of interaction: F6.039, 69.448 = 0.787, p = 0.584; effect of genotypes: F2, 23 = 3.027, p = 0.068) tasks. Post hoc Bonferroni corrections of the overall comparisons between genotypes and the comparisons for each session between genotypes were performed. See online supplementary table 1 for detailed statistical information on the two-way, one-repeated-measure ANOVA.
Fig. 5.
Latencies to collect the reward when mice chose the correct stimulus in the operant conditioning (a), VD (b), and VDR (c) tasks. Marks and error bars represent the mean ± SEM. WT mice: n = 9; D2L-Het mice: n = 7; D2L-KO mice: n = 10. n.s. = Not significant. Two-way, one-repeated-measure ANOVA showed no significant effects of genotypes and the interaction in the operant conditioning task (a; effect of interaction: F4.184, 48.111 = 0.420, p = 0.801; effect of genotypes: F2, 23 = 2.928, p = 0.074), significant effects of genotypes but not the interaction in the VD task (b; effect of interaction: F5.546, 63.781 = 2.289, p = 0.050; effect of genotypes: F2, 23 = 3.755, p = 0.039), and no significant effects of genotypes and the interaction in the VDR task (c; effect of interaction: F9.227, 106.110 = 0.756, p = 0.660; effect of genotypes: F2, 23 = 2.728, p = 0.087). Post hoc Bonferroni corrections of the overall comparisons between genotypes were performed (* p < 0.05). The comparisons for each session between genotypes were also performed with Bonferroni correction for the number of sessions (KO vs. WT: # p < 0.05/5). See online supplementary table 1 for detailed statistical information on the two-way, one-repeated-measure ANOVA.
Discussion
In this study, D2L-KO mice were impaired in visual discrimination and reversal learning in the VD and VDR tasks. The deficit in reversal learning might be due to the low flexibility of learning in D2L-KO mice. Alternatively, the behavioral deficits seen in the VD and VDR tasks might be due to defective learning of a new CS-reward association during the VD and VDR tasks. D2L-KO mice also needed more prolonged training with frequent errors initially, suggesting that this might be a reason for the slower acquisition in the VD and VDR tasks. The current finding is similar to previous reports of reversal learning impairments in patients with schizophrenia [7,9] and their mouse models [33]; however, the impairments were more pronounced in our study.
D2L receptors are expressed within the prefrontal cortex, striatum, and substantia nigra of the corticostriatothalamic circuit [16,44,45,46,47]. In the prefrontal cortex, D2Rs are primarily located in the pyramidal cells, and they control not only motor activity but also memory formation, attention, flexible behavior, and risk-based decision-making [48,49,50,51]. Thus, D2L receptors in the prefrontal cortex may be important for visual discrimination.
In the striatum, D2L receptors postsynaptically influence neural activity and plasticity in a specific population of projection neurons [14,52,53]. D2R-expressing neurons in the ventral striatum have critical roles in aversive learning and learning flexibility [43,54,55]. D2R-expressing neurons in the dorsolateral striatum play an essential role in the regulation of the correct response accuracy of conditional audio discrimination [56]. Dopamine may influence learning and its flexibility via striatal D2L receptors.
In addition, D2L receptors may function as autoreceptors in the substantia nigra pars compacta [47]. Future studies employing region-specific manipulations of D2L receptors will likely provide further elucidation of the role of D2L and D2S receptors in visual discrimination and reversal learning.
In conclusion, these results demonstrate a crucial role for D2L receptors in visual discrimination learning, and provide new insights into the molecular and circuit mechanisms underlying symptoms of neuropsychiatric disorders.
Statement of Ethics
All animal handling procedures were approved by the animal research committees of Kyoto University Graduate School of Medicine.
Disclosure Statement
The authors declare that there are no conflicts of interest regarding this paper.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
We thank Drs. T. Macpherson and T. Sakurai for critical reading and comments, N. Otani, K. Hirai, and Y. Miyoshi for technical support, Y. Nakanishi for preparing the manuscript, and A. Uto for preparing figures. This work was performed at the Open Innovation Laboratory for Drug Discovery and Development by Takeda and Kyoto University's ‘Basic and Clinical Research Project for CNS Drugs’ supported in part by Takeda Pharmaceutical Company Ltd. T.H. is supported by a Grant-In-Aid for Scientific Research on Innovative Areas (KAKENHI grant No. 23120011 and 16H06568) from the MEXT in Japan, JSPS KAKENHI grant No. 15H04275 and 16K14579, and grants from the Takeda Science Foundation, the Smoking Research Foundation, and the Daiichi-Sankyo Foundation of Life Science. In addition, A.S. is supported by the NIH (MH-084018, MH-094268 Silvio O. Conte Center, MH-069853, MH-085226, MH-088753, and MH-092443), Y.W. is supported by a Trust-Private fund, and T.S. is supported by the JSPS KAKENHI grant No. 26290029.
References
- 1.Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 2.Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- 3.Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman M, Oscar-Berman M. Spatial and visual learning deficits in Alzheimer's and Parkinson's disease. Brain Cogn. 1989;11:114–126. doi: 10.1016/0278-2626(89)90009-2. [DOI] [PubMed] [Google Scholar]
- 6.Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- 7.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lönnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282C:248–257. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnsten AFT, Wang M, Paspalas CD. Dopamine's actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol Rev. 2015;67:681–696. doi: 10.1124/pr.115.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macpherson T, Morita M, Hikida T. Striatal direct and indirect pathways control decision-making behavior. Front Psychol. 2014;5:1301. doi: 10.3389/fpsyg.2014.01301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi S, Hikida T, Yawata S. Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience. 2014;282C:49–59. doi: 10.1016/j.neuroscience.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- 16.Khan ZU, Mrzljak L, Gutierrez A, De la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci USA. 1998;95:7731–7736. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 18.Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hökfelt T, Borrelli E, Fisone G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci USA. 2003;100:4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaalund SS, Newburn EN, Ye T, Tao R, Li C, Deep-Soboslay A, Herman MM, Hyde TM, Weinberger DR, Lipska BK, Kleinman JE. Contrasting changes in DRD1 and DRD2 splice variant expression in schizophrenia and affective disorders, and associations with SNPs in postmortem brain. Mol Psychiatry. 2014;19:1258–1266. doi: 10.1038/mp.2013.165. [DOI] [PubMed] [Google Scholar]
- 20.Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 22.Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto PL, Hiranita T, Xu M, Hursh SR, Grandy DK, Katz JL. Dopamine D2-like receptors and behavioral economics of food reinforcement. Neuropsychopharmacology. 2016;41:971–978. doi: 10.1038/npp.2015.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fetsko LA, Xu R, Wang Y. Effects of age and dopamine D2L receptor-deficiency on motor and learning functions. Neurobiol Aging. 2005;26:521–530. doi: 10.1016/j.neurobiolaging.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Vargas-Pérez H, Borrelli E, Díaz JL. Wheel running use in dopamine D2L receptor knockout mice. Neurosci Lett. 2004;366:172–175. doi: 10.1016/j.neulet.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Zürcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, McDonald TJ, Nathanielsz PW, Nijland MJ. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Methods. 2010;188:219–225. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nithianantharajah J, McKechanie AG, Stewart TJ, Johnstone M, Blackwood DH, St Clair D, Grant SG, Bussey TJ, Saksida LM. Bridging the translational divide: identical cognitive touchscreen testing in mice and humans carrying mutations in a disease-relevant homologous gene. Sci Rep. 2015;5:14613. doi: 10.1038/srep14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SR, Alsiö J, Oomen CA, Holmes A, Saksida LM, Bussey TJ. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–1984. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brigman JL, Ihne J, Saksida LM, Bussey TJ, Holmes A. Effects of subchronic phencyclidine (PCP) treatment on social behaviors, and operant discrimination and reversal learning in C57BL/6J mice. Front Behav Neurosci. 2009;3:2. doi: 10.3389/neuro.08.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, Kiselycznyk C, Schmitt W, Sanderson DJ, Rawlins JN, Saksida LM, Bussey TJ, Sprengel R, Bannerman D, Holmes A. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–1272. doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romberg C, Horner AE, Bussey TJ, Saksida LM. A touch screen-automated cognitive test battery reveals impaired attention, memory abnormalities, and increased response inhibition in the TgCRND8 mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34:731–744. doi: 10.1016/j.neurobiolaging.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, Lewis FC, Sarvi MS, Foley GM, Crawley JN. 16p11.2 deletion mice display cognitive deficits in touchscreen learning and novelty recognition tasks. Learn Mem. 2015;22:622–632. doi: 10.1101/lm.039602.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartlett MS. Properties of sufficiency and statistical tests. Proc Royal Soc London Series A. 1937;160:268–282. [Google Scholar]
- 37.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- 38.Mauchly JW. Significance test for sphericity of a normal n-variate distribution. Ann Math Stat. 1940;11:204–209. [Google Scholar]
- 39.Hranilovic D, Bucan M, Wang Y. Emotional response in dopamine D2L receptor-deficient mice. Behav Brain Res. 2008;195:246–250. doi: 10.1016/j.bbr.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vukhac KL, Sankoorikal EB, Wang Y. Dopamine D2L receptor- and age-related reduction in offensive aggression. Neuroreport. 2001;12:1035–1038. doi: 10.1097/00001756-200104170-00034. [DOI] [PubMed] [Google Scholar]
- 41.Bulwa ZB, Sharlin JA, Clark PJ, Bhattacharya TK, Kilby CN, Wang Y, Rhodes JS. Increased consumption of ethanol and sugar water in mice lacking the dopamine D2 long receptor. Alcohol. 2011;45:631–639. doi: 10.1016/j.alcohol.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JW, Fetsko LA, Xu R, Wang Y. Dopamine D2L receptor knockout mice display deficits in positive and negative reinforcing properties of morphine and in avoidance learning. Neuroscience. 2002;113:755–765. doi: 10.1016/s0306-4522(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 43.Hikida T, Yawata S, Yamaguchi T, Danjo T, Sasaoka T, Wang Y, Nakanishi S. Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc Natl Acad Sci USA. 2013;110:342–347. doi: 10.1073/pnas.1220358110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder LA, Roberts JL, Sealfon SC. Distribution of dopamine D2 receptor mRNA splice variants in the rat by solution hybridization/protection assay. Neurosci Lett. 1991;122:37–40. doi: 10.1016/0304-3940(91)90187-x. [DOI] [PubMed] [Google Scholar]
- 45.Neve KA, Neve RL, Fidel S, Janowsky A, Higgins GA. Increased abundance of alternatively spliced forms of D2 dopamine receptor mRNA after denervation. Proc Natl Acad Sci USA. 1991;88:2802–2806. doi: 10.1073/pnas.88.7.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothmond DA, Weickert CS, Webster MJ. Developmental changes in human dopamine neurotransmission: cortical receptors and terminators. BMC Neurosci. 2012;13:18. doi: 10.1186/1471-2202-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neve KA, Ford CP, Buck DC, Grandy DK, Neve RL, Phillips TJ. Normalizing dopamine D2 receptor-mediated responses in D2 null mutant mice by virus-mediated receptor restoration: comparing D2L and D2S. Neuroscience. 2013;248:479–487. doi: 10.1016/j.neuroscience.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnsten AFT, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Druzin MY, Kurzina NP, Malinina EP, Kozlov AP. The effects of local application of D2 selective dopaminergic drugs into the medial prefrontal cortex of rats in a delayed spatial choice task. Behav Brain Res. 2000;109:99–111. doi: 10.1016/s0166-4328(99)00166-7. [DOI] [PubMed] [Google Scholar]
- 50.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 51.St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Yawata S, Yamaguchi T, Danjo T, Hikida T, Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2012;109:12764–12769. doi: 10.1073/pnas.1210797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishizawa K, Fukabori R, Okada K, Kai N, Uchigashima M, Watanabe M, Shiota A, Ueda M, Tsutsui Y, Kobayashi K. Striatal indirect pathway contributes to selection accuracy of learned motor actions. J Neurosci. 2012;32:13421–13432. doi: 10.1523/JNEUROSCI.1969-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data