Class 3 Sema signal through NRP and plexin coreceptors in human DCs to induce actin reorganization and promote cellular migration.
Keywords: neuropilin-1 and -2, plexins, chemotaxis, polysialic acid
Abstract
Class 3 semaphorins (Semas) are soluble proteins that are well recognized for their role in guiding axonal migration during neuronal development. In the immune system, Sema3A has been shown to influence murine dendritic cell (DC) migration by signaling through a neuropilin (NRP)-1/plexin-A1 coreceptor axis. Potential roles for class 3 Semas in human DCs have yet to be described. We tested the hypothesis that Sema3A, -3C, and -3F, each with a unique NRP-1 and/or NRP-2 binding specificity, influence human DC migration. In this report, we find that although NRP-1 and NRP-2 are expressed in human immature DCs (imDCs), NRP-2 expression increases as cells mature further, whereas expression of NRP-1 declines dramatically. Elevated levels of RNA encoding plexin-A1 and -A3 are present in both imDCs and mature DC (mDCs), supporting the relevance of Sema/NRP/plexin signaling pathways in these cells. Sema3A, -3C, and -3F bind to human DCs, with Sema3F binding predominantly through NRP-2. The binding of these Semas leads to reorganization of actin filaments at the plasma membrane and increased transwell migration in the absence or presence of chemokine CCL19. Microfluidic chamber assays failed to demonstrate consistent changes in speed of Sema3C-treated DCs, suggesting increased cell deformability as a possible explanation for enhanced transwell migration. Although monocytes express RNA encoding Sema3A, -3C, and -3F, only RNA encoding Sema3C increases robustly during DC differentiation. These data suggest that Sema3A, -3C, and -3F, likely with coreceptors NRP-1, NRP-2, and plexin-A1 and/or -A3, promote migration and possibly other activities of human DCs during innate and adaptive immune responses.
Introduction
DCs play a central role in innate and adaptive immunity. Upon contact with foreign antigens, microbes, or inflammatory factors in peripheral tissue, imDCs differentiate more fully and migrate to secondary lymphoid organs, where they interact with lymphocytes to initiate a primary immune response [1]. To facilitate these sentinel and antigen-presenting functions, DCs are adept at migrating in response to external guidance cues and forming specific intercellular contacts. Numerous soluble and cell-associated proteins, such as selectins, integrins, and chemokines and their receptors, contribute to these DC characteristics [2].
Semas comprise a large family of phylogenetically conserved, membrane-bound and secreted proteins that play a role in cell migration, adhesion, and diverse additional biologic processes [3, 4]. Semas are best known for their roles in nervous system development, where they mediate chemorepulsive axonal guidance [5]. They are expressed in many organ systems, though, and are involved in vascular development, tumor growth, suppression and metastasis, osteoclastogenesis, and immune cell activity [3]. The molecular mechanisms of Sema signaling involve cytoskeletal rearrangement and altered integrin-based adhesion to other cells and to the extracellular matrix and are mediated by plexins, NRPs, and additional cell surface receptors [3]. Of the 8 Sema subclasses that have been distinguished on the basis of structural features and sequence similarities [6], secreted class 3 Semas (Sema3A–G) are unique in that they require binding to NRP-1 or NRP-2 receptors [7–9] before interacting with signal-transducing plexin coreceptors [10]. Plexins also comprise a large family of transmembrane receptors that are expressed on a wide variety of cells types [11]. Intracellular signaling through the plexins occurs via the Rho family of GTPases and tyrosine kinases [3].
NRP-1 and -2 are transmembrane glycoproteins whose short cytoplasmic tails preclude Sema-induced signaling but are essential coreceptors with plexins for class 3 Semas [7–9]. We previously reported that differentiation of monocytes into mDCs induces the expression of NRP-2 and that NRP-2 in mDCs is polysialylated [12]. NRP-1 has also been shown to be expressed on the surface of human imDCs [13]. In the absence of Sema ligands, NRP-1 and NRP-2, on the surface of DCs, have been reported to have opposite effects on DC–T lymphocyte interactions—whereas NRP-1 enhances DC–T lymphocyte synapse formation and immune activation [13], NRP-2 inhibits T lymphocyte activation and proliferation [12]. Although the polySia glycan of NRP-2 impairs the DC–T lymphocyte interaction, it serves a vital role in binding chemokine CCL21 and directing CCR7 receptor-induced DC migration [14–16].
Several Semas have been reported to influence DC activity via signaling through cell-surface plexin-A1 and additional Sema receptors [17, 18]. Sema6D, expressed on the surface of T cells, stimulates bone marrow-derived DCs to produce cytokine IL-12 and increases expression of MHC class II molecules after binding to plexin-A1 on the surface of DCs [19]. Sema4D is also expressed by T cells and promotes activation and maturation of DCs after binding the CD72 receptor on DCs [20]. Poxvirus-encoded SemaA39R binds plexin-C1 on DCs and inhibits integrin-mediated adhesion and spreading, as well as chemokine-induced migration of DCs [21]. More recently, secreted Sema3A has been shown to signal through the NRP-1/plexin-A1 axis to induce myosin light-chain phosphorylation and promote directed migration of murine DCs into the lymphatics [22]. The role of known Sema receptors in guiding cell activity is likely more complex than currently appreciated, as plexin-A1 on the surface of DCs mediates interaction with lymphocytes, even in the absence of Semas [23].
Whereas most of the studies of Semas in the immune system have focused on the role of membrane-associated forms in cell activation and adhesion, the potential function of secreted class 3 Semas in regulating migration of human DCs has not been reported. In this study, we report that human monocyte-derived DCs express plexin-A1 and -A3, as well as their obligate NRP-1 and NRP-2 coreceptors for secreted class 3 Semas. We demonstrate that the level of expression of NRP-1 and NRP-2 diverges as DCs mature. The continued increase in expression of NRP-2 in mDCs and decline in NRP-1 expression suggest a major role for NRP-2 in Sema/NRP/plexin-mediated signaling in these cells. We also demonstrate that binding of Sema3A, -3C, and -3F to human mDCs—the latter predominantly through NRP-2—changes dynamics of F-actin organization at the plasma membrane and promotes transwell migration in the absence or presence of a chemokine CCL19 gradient. The enhanced transwell migration of Sema-treated DCs does not appear to be a result of increased cell speed or a decrease in cell size, raising the possibility of increased cell deformability as a potential explanation. These data demonstrate that multiple class 3 Semas have the potential to use NRP and plexin coreceptor family members to promote migration and possibly other activities of human DCs during innate and adaptive immune responses.
MATERIALS AND METHODS
Differentiation of purified monocytes into DCs
Monocytes were purified from leukocytes acquired commercially (leukapheresis product from SeraCare Life Sciences, Milford, MA, USA) and from local donors (guided by a protocol approved by the University of Maryland School of Medicine Institutional Review Board, Baltimore, MD, USA) and were differentiated in culture into mDCs in the presence of IL-4 and GM-CSF (both from R&D Systems, Minneapolis, MN, USA) and LPS (Sigma-Aldrich, St. Louis, MO, USA), as described previously [12, 24].
Immunofluorescent staining of cell-surface proteins and analysis by flow cytometry and confocal microscopy
Proteins on the surface of intact monocytes, imDCs and mDCs, were detected by incubating cells at 1 × 106 cells/ml in PBS containing 2% heat-inactivated human serum (Gemini Bio-Products, Calabasas, CA, USA) and anti-CD32 FcR IgG (1.5 μg/ml; Stem Cell Technologies, Vancouver, BC, Canada) before staining with 10 μg/ml mouse anti-NRP-2 IgG (C-9; Santa Cruz Biotechnology, Dallas, TX, USA) or anti-NRP-1 mAb (AD5-17F6; Miltenyi Biotec, Auburn, CA, USA). Bound IgGs were detected by incubation with biotinylated rabbit anti-mouse IgG, followed by PE-conjugated streptavidin (both from Dako, Carpinteria, CA, USA). Fluorescence was analyzed by flow cytometry using a BD FACSCalibur (BD Biosciences, Mountain View, CA, USA), and data were analyzed using FlowJo data analysis software (Tree Star, Ashland, OR, USA).
For visualization of NRP-1 and NRP-2 on the surface of mDCs, cells were stained with 10 μg/ml mouse anti-NRP-1 mAb (Miltenyi Biotec) and polyclonal goat anti-NRP-2 IgG (R&D Systems) for 1 h. Cells were washed in PBS and incubated for 1 additional h with Cy5-conjugated anti-goat IgG or with Cy2-conjugated anti-mouse IgG (both from Jackson ImmunoResearch Laboratories, West Grove, PA, USA). After washing in PBS, cells were fixed in 1% paraformaldehyde and seeded at 3 × 105 cells/well on poly-l-lysine-precoated, 8-chamber glass slides (Lab-Tek, Nunc; Nalge Nunc International, Rochester, NY, USA). Cell-associated fluorescence was visualized with a Zeiss LSM 510 Meta confocal system (Zeiss, Thornwood, NY, USA) using a C-Apo 40×/1.2 water-immersion objective after excitation at 488 or 633 nm.
Isolation of RNA and real-time RT-PCR
Total RNA was isolated from monocytes and monocyte-derived DCs using an RNeasy mini kit (Qiagen, Valencia, CA, USA), as described previously [12]. cDNA was prepared using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA), and semiquantitative real-time PCR was performed using the iQ SYBR Green Supermix kit (Bio-Rad Laboratories) with an ABI sequence detection system (ABI Prism 5700; Thermo Fisher Scientific, Waltham, MA, USA), as described previously [12]. Expression of the following genes (with GenBank accession numbers) was analyzed: NRP-1 (AF016050), NRP-2 (AF016098), Sema3A (NM_006080), Sema3B (NM_004636), Sema3C (NM_006379), Sema3D (NM_152754), Sema3E (NM_012431), Sema3F (NM_004186), Sema3G (NM_020163), plexin-A1 (NM_032242), plexin-A2 (NM_025179), plexin-A3 (NM_017514), plexin-A4 (NM_020911), plexin-D1 (NM_015103), VEGF-R1 (NM_002019.4), VEGF-R2 (NM_002253.2), and VEGF-R3 (NM_182925.4). Gene expression of GAPDH was also measured as an internal control. The following primers were selected using Primer-BLAST (National Center for Biotechnology Information, Bethesda, MD, USA) and were synthesized by Qiagen: Sema3A (forward, nt 100–120, 5′-GTGCCAAGGCTGAAATTATCCT-3′, and reverse, nt 327–305, 5′-CTTGCATTCATCTCTTCTGGTGT-3′) yielding a 227-bp product; Sema3B (forward, nt 656–587, 5′-ACATTGGTACTGAGTGCATGAAC-3′, and reverse, nt 679–660, 5′-CCCACTTCCACAAAGGCACA-3′) yielding a 114-bp product; Sema3C (forward, nt 578–659, 5′-TTTGCGTGTTGGTTGGAGTAT-3′, and reverse, nt 728–706, 5′-TCCTGTAGTCTAAAGGATGGTGG-3′) yielding a 150-bp product; Sema3D (forward, nt 1251–1271, 5′-ACCCACTGATTAAGTCCACCC-3′, and reverse, nt 1467–1445, 5′-ACAGTTCCAATGTCTGTTCCAAG-3′) yielding a 216-bp product; Sema3E (forward, nt 561–582, 5′-GGTTACGCCTGTCACATAAAGA-3′, and reverse, nt 760–740, 5′-TGTACTCGGCCAGTGTATCTC-3′) yielding a 200-bp product; Sema3F (forward, nt 1232–1250, 5′-AGCAGACCCAGGACGTGAG-3′, and reverse, nt 1346–1326, 5′-AAGACCATGCGAATATCAGCC-3′) yielding a 114-bp product; Sema3G (forward, nt 179–201, 5′-CCATGTACCTAGATGAGTACCGA-3′, and reverse, nt 357–340, 5′-CGAAGTTGGCGCACTCTGT-3′) yielding a 178-bp product; plexin-A1 (forward, nt 8053–8072, 5′-CACATGGCTGTGTCTAGGCG-3′, and reverse, nt 8175–8156, 5′-GCAGAGGGAGGTACGGAGTA-3′) yielding a 104-bp product; plexin-A2 (forward, nt 4–25, 5′-GATTTGGTCGTCTCCCTGATTC-3′, and reverse, nt 148–129, 5′-GGTTGGCCCTCACATGATTC-3′) yielding a 145-bp product; plexin-A3 forward, nt 2054-2073, 5′-CCAACGGGGATCAGGAGGT-3′, and reverse, nt 2262-2244, 5′-CGTTGACACCTGACAGATAGT-3′) yielding a 208-bp product; plexin-A4 (forward, nt 728–748, 5′-GCTCCTCATAGACTACAAGGA-3′, and reverse, nt 870–850, 5′-CGTTGACACCTGACAGATAGT-3′) yielding a 142-bp product; plexin-D1 (forward, nt 2076–2096, 5′-CATGGAGATGGCCTGTGACTA-3′, and reverse, nt 2197–2179, 5′-GGAAGGGCGGAAACTGGTC-3′) yielding a 122-bp product; VEGF-R1 (forward, nt 3484–3504, 5′-GCTCCCGAATCTATCTTTGAC-3′, and reverse, nt 4132–4112, 5′-CCGACTCCTTACTTTTACTGG-3′) yielding a 648-bp product; VEGF-R2 (forward, nt 490–510, 5′-GGCCCAATAATCAGAGTGGCA-3′ and reverse, nt 594–572, 5′-TGTCATTTCCGATCACTTTTGGA-3′) yielding a 104-bp product; VEGF-R3 (forward, nt 2302–2320, 5′-GGATGCGGGACGCTATCTG-3′, and reverse, nt 2420–2400 5′-CGACAAGGATCACGATCTCCA-3′) yielding a 118-bp product. All reactions were run under conditions described previously [12].
Production of AP-Sema3A, AP-Sema3C, and AP-Sema3F and DC-binding assay
Human Sema3A and Sema3F fusion proteins, containing an AP tag at the NH2 terminus (AP-Sema3A and AP-Sema3F) and an AP tag control, were expressed in HEK293T cells that were transfected with Sema-pAPtag-5 DNA constructs (kindly provided by Dr. David Ginty, Harvard Medical School, Cambridge, MA, USA), using Lipofectamine Plus (Thermo Fisher Scientific). Murine Sema3C, fused to an AP NH2 terminus tag, was similarly expressed in HEK293T cells using an AP-Sema3C pCDNA1 construct (kindly provided by Dr. Alex Kolodkin, Johns Hopkins School of Medicine, Baltimore, MD, USA). Secreted AP-Sema3A, AP-Sema3C, AP-Sema3F, and lone AP (from empty control vector) were concentrated from the medium (RPMI 1640 medium containing 5% fetal bovine serum) of transfected cells using Amicon Ultra-15 centrifugal filters (EMD Millipore, Danvers, MA, USA). The concentration of each Sema was determined by comparison with a standard curve of AP activity, generated using known amounts of human placental AP (Sigma-Aldrich). Binding of AP-Sema3A, AP-Sema3C, AP-Sema3F, and lone AP to the surface of mDC was measured after adding 2.5 µg of each ligand to 5 × 105 cells in 100 µl PBS/0.1% BSA and incubating the reaction at 4°C for 30 min. After washing DC once with PBS, cells were resuspended in 100 µl PBS, 50 µl was added to 2 wells of a 96 microtiter plate (Corning Costar; Sigma-Aldrich), and 200 µl AP substrate p-nitrophenylphosphate (Sigma-Aldrich) was added to each well. The colorimetric reaction was stopped by adding 50 µl 3 N NaOH, and absorbance was read at 405 nm with a Victor2 1420 spectrofluorometer (Wallac, Turku, Finland). Where indicated, DCs were preincubated with goat preimmune IgG or goat polyclonal anti-NRP-2 IgG (both from R&D Systems) at 40 µg/ml for 30 min at 37°C before adding Semas. This concentration of antibody was used, as no further decrease in Sema3F binding occurred at higher concentrations. In some experiments, cells were first treated with endoN (kindly provided by Karen Colley, University of Illinois, Chicago, IL, USA), which specifically cleaves α2-8-linked polySia.
Visualization of F-actin in Sema-treated mDCs with phalloidin by confocal microscopy
mDCs were harvested and seeded at 4 × 105 cells/well in phenol red-free RPMI 1640 medium containing 5% FCS on poly-l-lysine-precoated, 8-chamber glass slides (Lab-Tek, Nunc; Nalge Nunc International), incubated with 10 µg/ml AP-Sema3A, AP-Sema3F, or AP control and with 2.5 µg/ml human IgG1 control or 10 µg/ml human Sema3C-Fc (both from R&D Systems) for 60 min, fixed with 1% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained with phalloidin conjugated to either Alexa Fluor 488 (Thermo Fisher Scientific; for Sema3A- and Sema3F-treated cells) or to tetramethylrhodamine B isothiocyanate (Sigma-Aldrich; for Sema3C-treated cells). Cell-associated fluorescence was visualized with a Zeiss LSM 510 Meta confocal system using a C-Apo 40×/1.2 water-immersion objective after excitation at 488 or 647 nm and analyzed with either LSM 510 Meta acquisition software (Sema3A- and Sema3F-treated cells) or with Zen 2009 acquisition software (Sema3C-treated cells). The diameter of Sema-treated DCs was measured from photomicrographs of phalloidin-stained cells using LSM 5 Image Browser Software (Zeiss).
Transwell migration of DCs
Quantitative transwell migration assays were performed using QCM chemotaxis 96-well migration kits (EMD Millipore) with a 5 µm pore size, according to the manufacturer’s instructions. mDCs (4 × 104 cells) were exposed to 10 µg/ml AP-Sema3A, AP-Sema3F, or AP control and to 2.5 µg/ml human IgG1 control or 10 µg/ml human Sema3C-Fc in 100 μl RPMI 1640/0.5% BSA in a transwell insert that was placed into the lower chamber containing 150 µl RPMI 1640/0.5% BSA, with or without 200 µg/ml chemokine CCL19 (R&D Systems). Where indicated, DCs were preincubated with goat preimmune IgG (R&D Systems) or goat polyclonal anti-NRP-2 IgG (R&D Systems) at 40 µg/ml for 30 min at 37°C before adding Semas. After a 3 h incubation at 37°C in 5% CO2, cells that had migrated into the lower chamber and that remained attached to the bottom of the filter were collected, lysed, and stained with CyQuant GR dye, as suggested by the manufacturer, and quantified by recording fluorescence on an HTS 7000 Bio Assay reader with a 480/520 nm filter set (PerkinElmer, Waltham, MA, USA). All assays were performed in duplicate. A standard curve for fluorescence and number of DCs was established for each experiment, from which the number of migratory cells was interpolated for comparison with the number of cells placed in the upper chamber.
Microfluidic-based microchannel assay to study the migration of mDCs
Polydimethylsiloxane microchannels devices (400 µm long, 10 µm high, and 6 µm wide) were fabricated using a photolithography and a standard replica molding technique, as described previously [25–27]. The devices were coated with 20 μg/ml fibronectin (Sigma-Aldrich) to facilitate binding of mDCs. Before cell seeding, mDCs were harvested and left untreated or incubated with 2.5 µg/ml human IgG1 control or 10 µg/ml Sema3C-Fc for at least 1 h. Cells were collected and resuspended at 1 × 107 cells/ml in RPMI 1640 medium containing 1% FCS, and 10 μl of the cell suspension (equivalent to a total of 1 × 105 cells) was added to the cell inlet wells. After a 10 min incubation to allow cells to adhere, media in the inlet wells was removed and replaced with fresh RPMI 1640/1% FCS, and chemoattractant CCL19 at 100 ng/ml in RPMI 1640/1% FCS was added to the top distal wells. Migration of mDCs into the microchannels was visualized and recorded with time-lapse live microscopy in an enclosed and humidified microscope stage maintained at 37°C and 5% CO2 using software-controlled stage automation (inverted Eclipse Ti microscope; Nikon, Melville, NY, USA). Phase-contrast images were taken at 10 min intervals for a total duration of 24 h with a 10× Ph1 objective. Cells migrating in the first 10 h of the experiments were analyzed using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA) with the MTrackJ plugin. Motility parameters, such as speed (defined as the total path traveled by the cell per unit time during the first 10 h of the migration experiments), velocity (defined as the net cell displacement per unit time during the 10 h time window), and persistence (defined as the ratio of the net cell displacement to the total path traveled by the cell), were computed from the cell tracks data generated. The experiments were performed and analyzed in a blinded manner to eliminate observer bias.
RESULTS
Expression of NRP-1 and -2 and type A plexins in human DCs
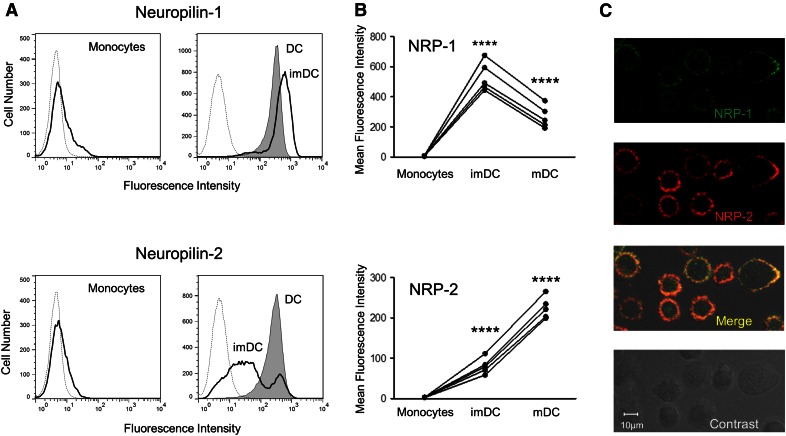
We previously reported that expression of NRP-2 is induced de novo in human imDCs and is markedly up-regulated at mRNA and protein levels during further cell maturation (Fig. 1A, lower) [12]. To determine whether the pattern of expression of NRP-1, the other member of the NRP receptor family, parallels that of NRP-2, we compared the change in protein and mRNA levels of each during differentiation of monocytes to DCs. Although not detected on the surface of monocytes by flow cytometry, NRP-1 expression increases during differentiation of human monocytes into imDCs (Fig. 1A, upper) [13]. In contrast to NRP-2, though, the amount NRP-1 on the cell surface declines following exposure to LPS and further maturation into mDCs (Fig. 1A). Although the absolute amounts of NRP-1 and NRP-2 on the surface of imDCs and mDCs from multiple donors vary, the dissimilar patterns of expression of each were repeatedly detected (Fig. 1B and Supplemental Table 1). The change in expression of cell-surface NRP-1 and NRP-2 during monocyte differentiation corresponds to the change in levels of mRNA encoding each protein. Whereas the amount of mRNA encoding NRP-2 continues to increase following exposure of imDCs to LPS, there is a dramatic decline in mRNA encoding NRP-1 as imDCs mature further (Fig. 2A and Table 1). In spite of this divergent pattern in NRP-1 and NRP-2 protein and mRNA expression that occurs 2 d following LPS stimulation, significant amounts of both proteins are detected on the surface of mDCs by flow cytometry and confocal microscopy (Fig. 1A and C). Thus, mDCs have the potential of responding via both NRP receptors to numerous environmental cues transmitted by the family of class 3 Sema signaling molecules.
Figure 1. Dissimilar patterns of expression of NRP-1 and NRP-2 during differentiation of DC.
The amount of NRP-1 (A, upper) and NRP-2 (A, lower) on the surface of intact human monocytes (solid lines, left), imDCs (solid lines, right), and mDCs (shaded regions, right) was determined by flow cytometry after cells were stained with anti-NRP-1 or anti-NRP-2 IgGs or isotype control IgG (dotted lines, left and right). The mean fluorescence intensity of staining for NRP-1 and NRP-2 on monocytes, imDCs, and mDCs from 5 donors is shown (B). The change in average mean fluorescence intensity of imDCs compared with monocytes and of mDCs compared with imDCs, shown from 5 donors, is significant (****P < 0.0001). Surface expression of NRP-1 (C, top) and NRP-2 (C, second from top) on mDCs is shown by confocal microscopy. Bleed-through for red and green dyes was checked before acquiring data to secure color separation. The results shown in A from 1 donor and in B from 5 donors are representative of data from 7 different donors (all shown in Supplemental Table 1), and the micrographs in C are representative of staining of mDCs from 3 different donors.
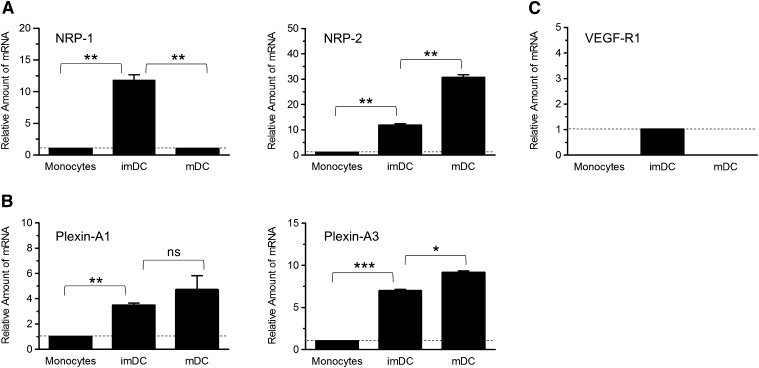
Figure 2. Change in expression of mRNAs encoding NRP-1 and NRP-2, plexin-A1 and -A3, and VEGF-R1 during differentiation of monocytes into imDCs and mDCs.
Total RNA was isolated from monocytes and monocyte-derived imDCs and mDCs and was analyzed for expression of genes encoding NRP-1 and NRP-2 (A), plexin-A1 and -A3 (B), and VEGF-R1 (C) by SYBR Green semiquantitative real-time RT-PCR, as described in Materials and Methods. The fold change in each mRNA in imDCs and mDCs compared with monocytes (or compared with imDCs when no RNA was detected in monocytes) is shown relative to the change in the expression of GAPDH RNA. When RNA encoding a gene was detected in monocytes, the level of expression was set to 1, as noted by the dotted, horizontal lines. When no RNA encoding a gene was detected in monocytes, the level detected in imDCs was set to 1. Data represent the means ± se of samples run in triplicate and are representative of data from experiments using cells from 3 different donors, as described in Table 1 [*P < 0.05; **P < 0.01; ***P < 0.001; not significant (ns), P > 0.05].
TABLE 1.
Gene expression of NRPs and plexins in human monocytes and DCs
| Donor | Gene | Relative amount of mRNA for each gene |
||
|---|---|---|---|---|
| Monocytes | imDCs | mDCs | ||
| 1 | GAPDH | (20.41) | (17.23) | (17.61) |
| NRP-1 | (26.69) | 8.61 ± 0.18 (20.41) | 0.19 ± 0.00 (26.30) | |
| NRP-2 | (30.00) | 28.96 ± 6.80 (22.00) | 96.47 ± 20.10 (20.63) | |
| PLX-A1 | (29.21) | 4.98 ± 0.09 (23.71) | 3.52 ± 0.15 (24.59) | |
| PLX-A3 | (32.54) | 5.72 ± 0.02 (26.84) | 12.84 ± 0.84 (26.05) | |
| 2 | GAPDH | (19.80) | (18.00) | (17.59) |
| NRP-1 | (26.02) | 11.86 ± 1.82 (20.65) | 1.03 ± 0.04 (24.27) | |
| NRP-2 | (27.24) | 11.77 ± 0.99 (21.88) | 30.61 ± 1.47 (20.6) | |
| PLX-A1 | (28.42) | 3.49 ± 0.17 (24.82) | 4.41 ± 1.43 (24.65) | |
| PLX-A3 | (31.79) | 6.97 ± 0.19 (27.19) | 9.16 ± 0.19 (26.89) | |
| 3 | GAPDH | (18.79) | (17.92) | (17.55) |
| NRP-1 | (25.96) | 17.51 ± 5.84 (21.27) | 0.30 ± 0.11 (29.04) | |
| NRP-2 | (27.73) | 3.61 ± 1.13 (24.75) | 33.83 ± 0.59 (23.32) | |
| PLX-A1 | (29.01) | 1.44 ± 0.45 (27.27) | 0.82 ± 0.14 (29.89) | |
| PLX-A3 | (31.86) | 2.50 ± 01.05 (29.90) | 3.97 ± 1.38 (31.06) | |
Monocytes, monocyte-derived imDCs, and mDCs from 3 different donors were analyzed for NRP-1, NRP-2, plexin (PLX)-A1, and plexin-A3 mRNA expression. The fold increase in level of mRNA encoding each protein is indicated, with the PCR crossover threshold value for the amount of each mRNA encoding each protein noted in parentheses. Fold increase in mRNA in imDCs and mDCs is based on the level in monocytes and is normalized to the change in GAPDH mRNA. No mRNA encoding plexin-A2, -A4, or -D1 was detected in all 3 donors.
As NRP-1 and NRP-2 are coreceptors with type A plexins or VEGF-Rs, depending on the type of cell in which they are expressed, we determined whether members of each coreceptor family are expressed in monocytes, imDCs, and mDCs and thus, have the potential for forming a signaling complex with NRP-1 and/or NRP-2 in these cells. The amount of mRNA encoding plexins A1–A4, plexin-D1, and VEGF-R1–3 in monocytes and monocyte-derived cells was determined, and the change in the amount of each during differentiation was normalized to expression of GAPDH RNA. Although a relatively low level of RNA encoding plexin-A1 and -A3 was detected in freshly isolated monocytes, the expression of each was up-regulated in imDCs and remained elevated after cells were exposed to LPS (Fig. 2B and Table 1). No RNA encoding plexin-A2 and -A4 or plexin-D1, reported to interact with class 3 Semas [28], were detected in monocytes or DCs (results not shown). Analysis of RNAs encoding VEGF-R1, VEGF-R2, and VEGF-R3 in monocytes and in monocyte-derived DCs showed transient expression of VEGF-R1 only in imDCs (Fig. 2C), whereas both VEGF-R2 and VEGF-R3 were not detected in monocytes at any stage of differentiation (unpublished results). These data suggest that in the presence of class 3 Semas, NRP-1 and NRP-2 on the surface of DCs can potentially associate with plexin-A1 and/or -A3 to transmit intracellular signals.
Human DCs up-regulate expression of Sema3C
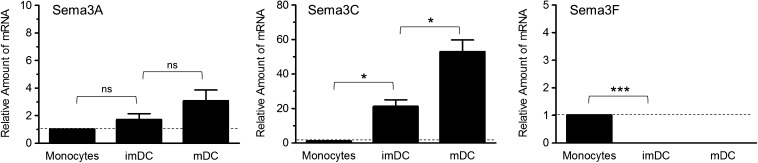
Although human DCs express NRP-1, NRP-2, and plexin-A1 and -A2 coreceptors, definitive sources of physiologically relevant class 3 Sema ligands remain to be identified. To determine whether monocytes and monocyte-derived DCs synthesize the Sema ligands for their NRP/plexin coreceptors, the amount of mRNA encoding all secreted class 3 Semas in these cells was quantitated. Sema3C was unique among the class 3 Semas, with mRNA encoding this gene present in monocytes and significantly up-regulated in imDCs and mDCs (Fig. 3 and Table 2). In contrast, although no mRNA encoding Sema3A was detected in monocytes, Sema3A mRNA was present in imDCs and mDCs, albeit with variable patterns of expression among different donors (Fig. 3 and Table 2). The expression of Sema3F in monocytes and derived cells differed from that of Sema3A and -3C. mRNA encoding Sema3F was present in monocytes but was down-regulated to undetectable in imDCs and DCs. No mRNA encoding Sema3B, -3D, -3E, or -3G was detected in all donors. In spite of the presence of mRNA encoding Sema3A, -3C, and -3F in monocytes or imDCs and mDCs, attempts to detect secreted Semas in concentrated culture medium of these cells or cell-associated Semas in cell lysates on immunoblot were unsuccessful (data not shown), suggesting a relatively low concentration of synthesized protein.
Figure 3. Change in expression of mRNAs encoding class 3 Semas during differentiation of monocytes into imDCs and mDCs.
Total RNA was isolated from monocytes and monocyte-derived imDCs and mDCs and was analyzed for expression of genes encoding all class 3 Semas by SYBR Green semiquantitative real-time RT-PCR, as described in Materials and Methods. The fold change in each mRNA in imDCs and mDCs compared with monocytes is shown relative to the change in the expression of GAPDH RNA. The level of expression of each gene in monocytes was set to 1, as noted by the dotted, horizontal lines. Data represent the means ± se of samples run in triplicate and are representative of data from experiments using cells from 3 different donors, as seen in Table 2 (*P < 0.05; ***P < 0.001; ns, P > 0.05).
TABLE 2.
Gene expression of class 3 Semas in human monocytes and DCs
| Donor | Gene | Relative amount of mRNA for each gene |
||
|---|---|---|---|---|
| Monocytes | imDCs | mDCs | ||
| 1 | GAPDH | (20.41) | (17.23) | (17.61) |
| Sema3A | (30.61) | 0.18 ± 0.01 (29.87) | 0.53 ± 0.06 (28.74) | |
| Sema3C | (28.61) | 12.68 ± 0.00 (21.77) | 25.4 ± 2.46 (21.15) | |
| Sema3F | (26.23) | 0.00 ± 0.00 (31.30) | 0.00 ± 0.00 (32.21) | |
| 2 | GAPDH | (19.80) | (18.00) | (18.09) |
| Sema3A | (31.39) | 1.71 ± 0.64 (28.92) | 3.07 ± 1.18 (28.17) | |
| Sema3C | (27.70) | 12.08 ± 4.04 (22.03) | 52.93 ± 7.83 (20.28) | |
| Sema3F | (26.42) | 0.01 ± 0.00 (31.24) | 0.01 ± 0.00 (31.88) | |
| 3 | GAPDH | (18.79) | (17.92) | (17.55) |
| Sema3A | (31.41) | 7.00 ± 2.61 (27.98) | 7.45 ± 2.23 (29.72) | |
| Sema3C | (27.73) | 23.46 ± 8.32 (22.54) | 67.30 ± 8.81 (23.13) | |
| Sema3F | (26.39) | 0.02 ± 0.00 (31.76) | 0.05 ± 0.25 (32.75) | |
Monocytes, monocyte-derived imDCs, and mDCs from 3 different donors were analyzed for class 3 Sema expression. The fold increase in level of mRNA encoding each Sema is indicated, with the PCR crossover threshold value for the amount of mRNA encoding each protein noted in parentheses. Fold increase in mRNA in imDCs and DCs is based on the level in monocytes and normalized to the change in GAPDH mRNA. No mRNA encoding Sema3B, -3D, -3E, or -3G was detected in all 3 donors.
Sema3A, -3C, and -3F bind to the surface of human imDCs and mDCs
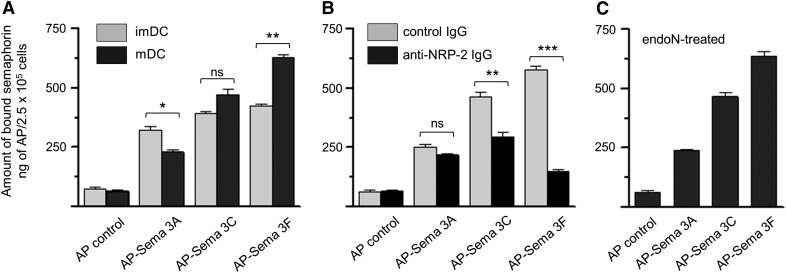
Given the presence of NRP-1 and NRP-2 on the surface of human DCs and the absence of detectable endogenous class 3 Sema ligands associated with these cultured cells, we tested the ability of exogenous, recombinant Sema3A (ligand for NRP-1), Sema3F (ligand for NRP-2), and Sema3C (ligand for both NRP-1 and NRP-2) to bind to these cells. All 3 of these Semas bind to the surface of human imDCs and mDCs (Fig. 4A). Whereas approximately equal amounts of Sema3A, -3C, and -3F bind to imDCs, the relative amount of each Sema bound to mDCs changes. There is relatively less Sema3A, but more Sema3C and -3F, bound to mDCs, consistent with the changes in surface expression of their respective NRP-1 and NRP-2 receptors. The binding of Sema3F to mDCs occurred predominantly via binding to NRP-2, as preincubation of mDCs with anti-NRP-2 IgG blocked >85% of Sema3F binding but reduced binding of Sema3A by only 15% (Fig. 4B). Sema3C binding to mDC was reduced 41% after cells were preincubated with anti-NRP-2 IgG, consistent with its known interaction with both NRP-1 and NRP-2. Removal of polySia, a glycan modification of NRP-2 that promotes chemokine CCL21 binding to NRP-2, had no detectable effect on the binding of Sema3A, -3C, or -3F to mDC (Fig. 4C compared with A). Thus, Sema ligands for both NRP-1 and NRP-2 bind to the surface of mDCs, with Sema3C binding partly, and Sema3F binding predominantly through NRP-2.
Figure 4. Sema3A, -3C, and -3F bind to the surface of human DCs.
(A) imDCs (gray bars) and mDCs (black bars) were incubated with recombinant AP-Sema3A, AP-Sema3C, AP-Sema3F, or lone AP (control), and binding of each ligand to the cell surface was determined by the amount of AP activity measured, as described in Materials and Methods. (B) mDCs were preincubated with preimmune goat IgG (control IgG; gray bars) or goat polyclonal anti-NRP-2 IgG (black bars) before exposure to Semas and subsequent measurement of bound AP activity. (C) mDCs were exposed to endoN to remove cell surface polySia before exposure to Semas and subsequent measurement of bound AP activity. In each experiment, each Sema (2.5 μg) was added to 5 × 105 cells, and following the incubation, the samples were divided in half, and AP activity was measured. Data are the average from 3 sets of duplicate wells ± se from 1 experiment and are representative of results for 3 different experiments (*P < 0.05; **P < 0.01; ***P < 0.001; ns, P > 0.05).
Sema3A, -3C, and -3F induce morphologic changes in mDCs
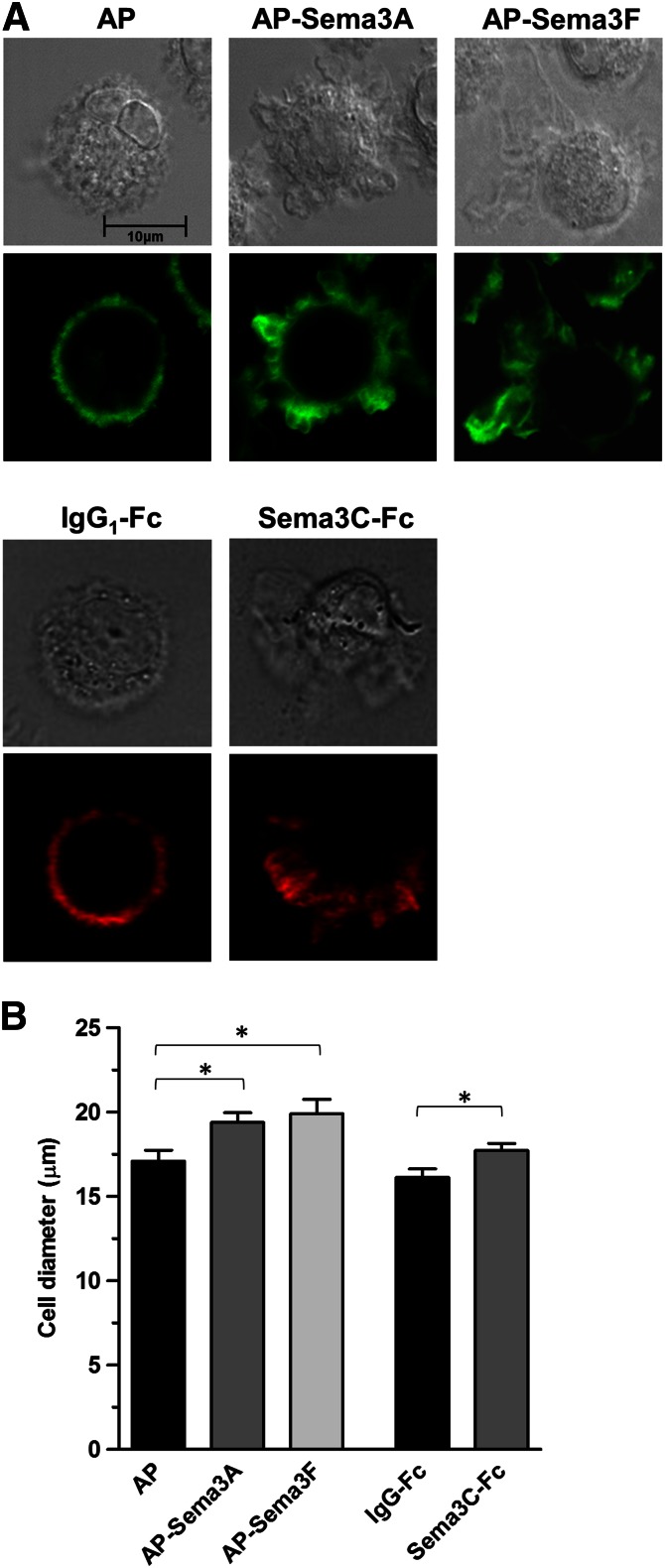
Although Sema3A has been shown to promote murine DC migration by inducing phosphorylation of myosin II via the NRP-1/plexin-A1 axis [22], the effect of Sema3A and of other class 3 Semas on human DC migration has not been evaluated. To determine whether Sema3A, -3C, and -3F affect the cytoskeletal arrangement in human DCs, a necessary step in cell motility, F-actin organization was visualized by confocal microscopy after DCs were exposed to each of these Semas and stained with fluorochrome-labeled phalloidin. Sema3A, -3F, and -3C were chosen for study to evaluate the effect of ligand binding to NRP-1, NRP-2, and both NRP-1 and NRP-2, respectively. Control cells were relatively round and clearly showed a uniform distribution of organized F-actin along the plasma membrane (Fig. 5A, left, AP and IgG1-Fc). In contrast, Sema3A and -3C (Fig. 5A, middle) and -3F (Fig. 5A, right) induced a marked reorganization of F-actin into focal areas coinciding with lamellae. Some DCs exposed to Sema3A, -3C, and -3F showed polarized distribution of F-actin (Fig. 5A, seen with Sema3F and -3C), suggesting cytoskeletal organization to promote directed migration.
Figure 5. Sema3A, -3C, and -3F induce F-actin rearrangement in mDCs.
(A) Cultured human mDCs were treated with AP-Sema3A, AP-Sema3F, or AP control and stained with phalloxin 488 nm (green; lower of upper panels) or were treated with Sema3C-Fc or human IgG1 control and stained with tetramethylrhodamine B isothiocyanate (red; lower of lower panels) to visualize filamentous F-actin fibers by confocal microscopy. Companion phase-contrast images are also shown (upper of upper and lower panels). Photomicrographs of DCs treated with the AP gene constructs and of cells exposed to the Fc chimeras are from experiments using cells from different donors and are representative of results using cells from 7 (for Sema3A and -3F) and 3 (for Sema3C) different donors. (B) The diameter of mDCs exposed to Sema3A, -3C, and -3F was determined from photomicrographs of stained cells using the LSM 5 Image Browser Software (Zeiss). Cell diameter was calculated from cells in 6 fields for each experimental condition. At least 25 ± 5 cells were counted in each field. Changes in cell diameter were significant in cells treated with AP-Sema3A, Sema3C-Fc, and AP-Sema3F compared with AP and IgG-Fc controls (*P < 0.05).
To quantitate the extent of change in cell morphology induced by Sema3A, -3C, and -3F, visualized in Fig. 5A, the change in size of Sema-treated cells was analyzed by LSM 5 Image Browser Software (Zeiss) after staining with fluorochrome-conjugated phalloidin. By including measurements from at least 125 cells per condition, this determination overcame the inherent imprecision of subjectively identifying Sema-treated cells with altered morphology compared with untreated control cells. All 3 Semas tested induced an average increase in diameter of cells in each population ranging from 10% to 16% of the size of control cells (Fig. 5B). Thus, although not able to determine the percentage of cells with altered morphology following exposure to class 3 Semas, the Sema-induced F-actin cytoskeletal rearrangements were extensive enough to increase significantly the average cell dimension calculated from a large sample of treated cells.
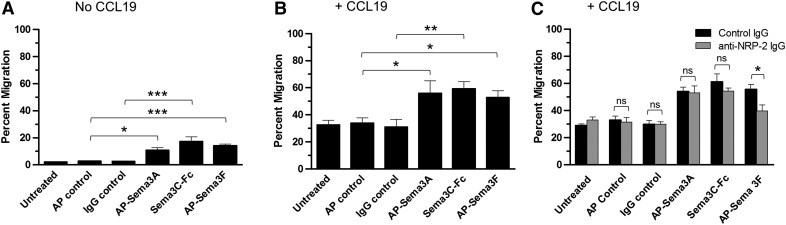
Sema 3A, -3C, and -3F promote the migration of human mDCs
To determine whether the cytoskeletal changes induced by Sema3A, -3C, and -3F in mDCs were associated with a change in migratory capacity, we evaluated the chemotactic activity of each Sema alone and in the presence of chemokine CCL19. When mDCs were treated with Sema3A, -3C, or -3F and placed in the top chamber of a transwell plate in the absence of CCL19, transwell migration of 10.9 ± 1.7, 17.3 ± 1.4, and 14.1 ± 1.2%, respectively, of input cells occurred (Fig. 6A). In each case, there were more migrating cells than the 2.2 ± 0.2% (AP control) and 2.8 ± 0.2% (IgG control) of input cells that migrated into the bottom chamber in the absence of these Semas. A similar pattern of enhanced migration was noted when chemoattractant CCL19 was present in the lower chamber. In the presence of CCL19 and Sema3A, -3C, or -3F, 56.0 ± 7.4, 59.3 ± 4.2, and 52.7 ± 4.3%, respectively, of added DCs migrated into the bottom chamber (Fig. 6B). In each case, the number was greater than the 33.9 ± 3.8% (AP control) and 30.9 ± 5.6% (IgG control) of cells that migrated in the presence of CCL19 alone.
Figure 6. Sema3A, -3C, and -3F promote mDC migration.
Migration of human monocyte-derived mDCs after exposure to AP-Sema3A, AP-Sema3F, or AP control and to Sema3C-Fc or human IgG1 was evaluated using quantitative transwell chemotaxis kits without chemokine CCL19 (A) or in the presence of CCL19 (B), as described in Materials and Methods. (C) mDCs were preincubated with preimmune goat IgG (control IgG, black bars) or goat polyclonal anti-NRP-2 IgG (gray bars) before exposure to Semas and subsequent measurement of migration. All assays were performed in duplicate, and percent migration represents the percentage of input DCs added to the upper chamber that migrated into the lower chamber. Data are from 1 experiment and are representative of results from 5 different experiments for A and B and from 3 different experiments for C (*P < 0.05; **P < 0.01; ***P < 0.001; ns, P > 0.05).
As we previously showed that anti-NRP-2 IgG blocked >85% of Sema3F and 41% of Sema3C binding to mDC, the effect of this antibody on transwell migration in the presence of these Semas was assessed. Under these conditions, the 1.7-fold increase in transwell migration of DCs treated with Sema3F was reduced by 74%, and the 1.9-fold increase in migration of Sema3C-treated cells declined by 24% (Fig. 6C). The blocking of NRP-2 had minimal impact on the migration of Sema3A-treated cells. These results suggest that Sema3A, -3C, and -3F induce F-actin reorganization and promote migration in the absence or presence of a chemokine gradient and that in the case of Sema3F, most of the enhanced migration is mediated through a NRP-2/plexin signaling axis.
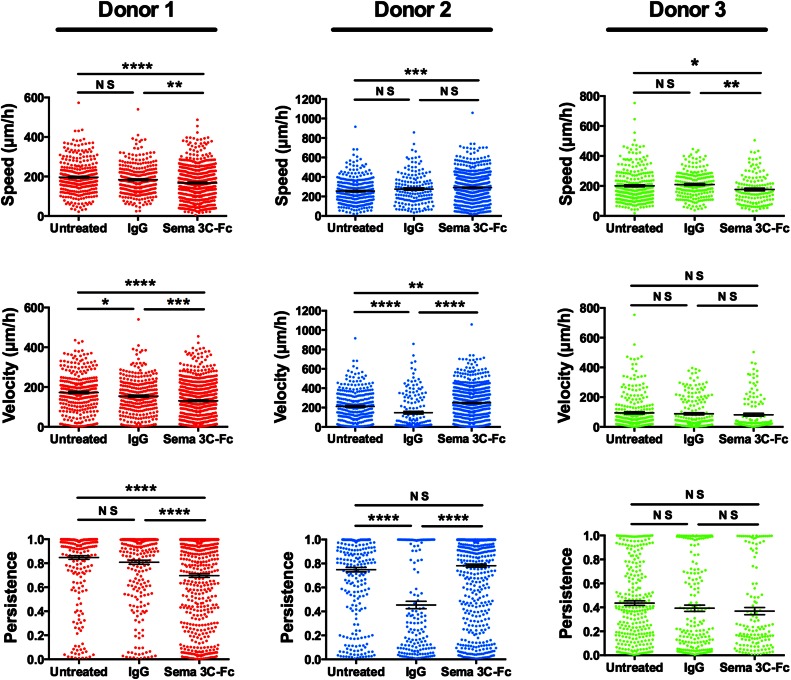
Sema3C has a variable effect on velocity of mDCs from different donors
It has been reported previously that exposure to Sema3A increases the speed and motility of murine DCs [22]. To determine whether this is a possible explanation for enhanced transwell migration of human DCs following exposure to Sema3A, -3C, and -3F (Fig. 6), the speed, velocity, and persistence of Sema3C-treated DCs were measured in a microfluidic-based microchannel assay. Sema3C was chosen as a representative class 3 Sema that signals through NRP-1 and NRP-2. Despite finding repeatedly that Sema3C enhanced transwell migration of DCs from multiple donors (Fig. 6), results of cell speed and motility were variable among all donors evaluated. Results from 1 donor that show no significant change in speed of Sema3C-Fc-treated cells compared with IgG control and from 2 other donors that reveal a small decline in speed of Sema3C-treated cells are shown in Fig. 7 and Supplemental Table 2. Likewise, the effect of Sema3C on persistence of mDCs from different donors is variable (Fig. 7 and Supplemental Table 2). The variable effect of Sema3C on motility of mDCs from multiple donors raises the possibility that Sema-induced signaling is donor dependent. Furthermore, these results suggest that enhanced speed and motility are not the predominant Sema-induced effects that explain the consistent enhancement in transwell migration.
Figure 7. Sema 3C has a variable effect on the speed, velocity, and persistence of mDCs from different donors.
Chemotaxis of human monocyte-derived mDCs that were untreated or treated with control IgG or with Sema3C-Fc was evaluated in a microfluidic-based microchannel assay, as described in Materials and Methods. Cell migration toward a CCL19 chemokine gradient was visualized and recorded by time-lapse live microscopy, and motility parameters, such as speed, velocity, and persistence were computed from the cell tracks data generated. Three independent experiments with mDCs, derived from 3 different donors, were conducted with Sema3C-Fc and are represented in the figure. Between 137 and 674 cells were analyzed for all conditions, as shown in Supplemental Table 2 (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, P > 0.05).
DISCUSSION
Semas comprise a diverse family of soluble and membrane-bound signaling proteins that mediate adhesion and migration of many different types of cells. In the immune system, Sema3A has been shown to promote migration of murine DCs into the lymphatics by signaling through NRP-1/plexin-A1 coreceptors [22]. In this report, we extend that finding by showing that Sema3C and -3F, as well as -3A, are capable of promoting migration of human DCs and that NRP-2, like NRP-1, is a functional coreceptor for class 3 Semas in these cells. We also show that Sema3A and -3F, ligands for NRP-1 and NRP-2, respectively, and Sema3C, which binds to both NRP-1 and NRP-2, induce F-actin reorganization in human DCs. As demonstrated in other cell types and likely the mechanism of action in human DCs, Sema signaling through Sema-binding NRP and signal-transducing plexin coreceptors affects actomyosin contractility by activating downstream GTPases [3] and by modulating phosphorylation of myosin light chain [22].
NRP-1 and -2 are necessary coreceptors with signal-transmitting plexins for soluble class 3 Semas. Although NRP-1 and NRP-2 have distinct spatiotemporal expression patterns and roles in cells of the CNS [9, 29], both are simultaneously expressed in human DCs. In the absence of Sema ligands, NRP-1 and NRP-2 have opposite effects on DC–T lymphocyte interactions [12, 13]. Whether these 2 NRP receptors, with seemingly similar functions, influence human DC activity cooperatively or independently in the presence of multiple potential Sema ligands remains to be determined. Murine DCs, like human DCs, express both NRP-1 [22] and NRP-2 [30, 31], yet NRP-1 and Sema3A were shown using NPR-1−/− mice to be indispensable for migration of murine DCs into the lymphatics [22]. These results imply that NRP-2 and its Sema ligands are not able independently to guide murine DC migration. They do not preclude, though, the possibility that both NRP-1 and NRP-2 are required to orchestrate Sema-induced migration. In fact, the silencing of the activity of NRP-2 in cortical axons that express both NRP-1 and NRP-2 changed the cellular response to Sema3C from attraction to repulsion [32]. Likewise, Sema3A and Sema3C were shown to act as antagonists of NRP-1 and agonists of NRP-2 in the CNS [33]. To add to the complexity of Sema signaling in mDCs, the expression of both plexin-A1 and -A3, along with NRP-1 and NRP-2 in mDCs, expands the coreceptor combinations that are possible. Although much remains to be learned about NRP-1 and NRP-2 coexpression in mDCs, the robust increase in expression of NRP-2 and decline in expression of NRP-1 during DC maturation that we find suggest an important role for NRP-2 in these cells.
In the CNS, class 3 Semas act as chemorepulsive agents causing collapse of neuronal growth cones [34, 35]. Likewise, Sema3A and Sema3F have been reported to be chemorepulsive for human thymocytes that express NRP-1 and NRP-2 in a transwell migration assay [36, 37], and Sema3A enhances chemokine-directed migration of murine DCs only when added to the upper chamber of a transwell unit, presumably by acting on the rear side of cells [22]. We show in this report that exposure of human mDCs to Sema3A, Sema3C, or Sema3F promotes migration from the upper into the lower chamber of a transwell unit in the absence or presence of a chemoattractive cytokine gradient. Although cells in each of these experiments migrated away from the Sema-containing upper chamber of a transwell unit, this movement is not necessarily a result of chemorepulsion. In fact, enhanced directed migration of human DCs occurs regardless of whether Sema3A, Sema3C, and Sema3F are added to upper or lower chambers of the transwell unit (data not shown). It is presumed that the concentration of Sema begins to equilibrate between upper and lower chambers of the transwell units during the course of the migration assay, making it difficult to attribute migration to repulsive or attractive factors. If indeed the effects of Semas on thymocytes, DCs, and neurons are different, it may be partly because of potential use of different plexin coreceptors with differing downstream signaling pathways in each type of cell, as discussed above, and/or from Sema-induced signaling through additional receptors remaining to be identified.
A previous study attributed Sema3A-enhanced transwell migration of murine DCs to an increase in cell speed and motility, resulting from phosphorylation of myosin II via the Sema3A/NRP-1/plexin-A1 signaling axis [22]. Despite the finding that Sema3A, Sema3C, and Sema3F consistently promote transwell migration of human mDCs from multiple donors, with the use of Sema3C as a representative class 3 Sema, we were not able to correlate this change in transwell migration with an increase in the average speed and/or velocity of mDCs. In fact, in 3 experiments with human mDCs from different donors, there was either no change or a small decrease in motility measurements in human DCs treated with human Sema3C-Fc. Preliminary optimization experiments conducted with human AP-Sema3A and AP-Sema3F and with murine AP-Sema3C using mDCs from 4 different donors also demonstrated inconsistent effects, with cells from some donors becoming more migratory upon Sema exposure, and cells from other donors becoming less migratory (data not shown). It is intriguing to speculate that these differences were donor dependent (e.g., cells expressed different relative amounts of NRP-1, NRP-2, plexin-A1, and plexin-A3), but this evaluation will require further study. The difference in our result and that reported for murine DCs [22] may be related to the unique features of the device used by the other group that is designed specifically to measure short-term effects of cell locomotion (within 20 min in that study) and the microfluidic chambers used in our studies that measure longer-term effects of cell movement (over 10 h). We also determined that Sema-treated DCs were larger than untreated cells, negating the possibility that a reduction in cell size might explain enhanced transwell migration through the 5 μ pores. Given the changes in F-actin organization, it is possible that increased deformability could explain enhanced transwell migration, as would be expected to occur at endothelial cell tight junctions.
Immune cell trafficking is orchestrated by a highly ordered network of chemokines/cytokines and adhesion molecules, such as integrins and selectins [38]. The significant contribution of class 3 Semas and their NRP and plexin coreceptors to this network of factors controlling DC migration in vivo is manifest by impaired migration of murine DCs into the lymphatics in the absence of Sema3A/NRP-1/plexin-A1 [22]. Directed migration of DCs in vivo likely depends not only on the relative expression of NRP-1 and NRP-2 in DCs but also on gradients of class 3 Semas that are formed at various anatomic sites. Although Sema3A and other class 3 Semas are expressed by murine lymphatic and vascular endothelial cells [22, 39, 40], their sites of expression, control of synthesis, and role in vivo remain to be determined. We have shown that mDCs express mRNA encoding Sema3A and Sema3C but are not clear whether these Semas function in an autocrine or paracrine manner. That exogenous Sema3A, Sema3C, and Sema3F bind to and induce a morphologic change in DCs suggests that the endogenous amounts may be relatively low. Alternatively, it is possible that the recombinant Semas that we have used in our experiments are qualitatively different from endogenous Semas that are known to be cleaved by metalloproteinases [41, 42]. The relative amounts of DC-derived Sema3A and Sema3C compared with class 3 Semas from others sources remain to be determined.
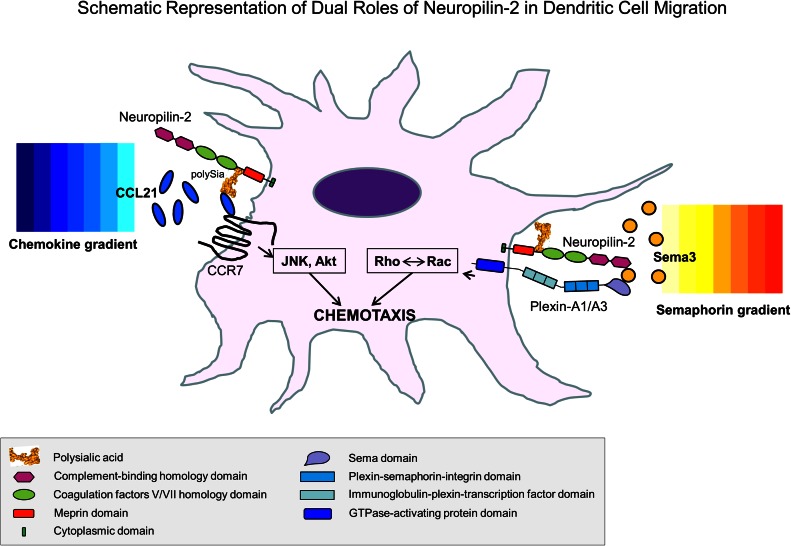
It is noteworthy that NRP-2 is a multifunctional protein in DCs that is capable of linking responses to both Semas and chemokines through its nonoverlapping Sema-binding domain and its post-translational modification with polySia [12, 14–16]. Whereas Sema3C and Sema3F bind to the N-terminus of NRP-2, polySia chains that are attached more distally to O-linked glycans on NRP-2 [30] have been shown to sequester chemokine CCL21 and to promote CCR7 receptor-mediated migration [14–16] (Fig. 8). Thus, NRP-2, whose expression is up-regulated during maturation of DCs [12, 31], can integrate signals from chemokines targeting movement with those from Semas facilitating cytoskeletal reorganization. The role of NRP-2 in DC migration may be even more complex, as NRPs on neighboring cells are also capable of forming homophilic and heterophilic interactions [7]. These multiple capacities of NRP-2 may be unique to the protein expressed in DCs, as NRP-2 is not polysialylated in other types of cells that have been analyzed (unpublished results). In addition, CCR7 has recently been reported to carry polySia [43], and the interaction between NRP-2 and CCR7 may be more complex than appreciated.
Figure 8. Schematic representation of dual roles of NRP-2 in DC migration.
The complement-binding domain of NRP-2 is shown facilitating binding of class 3 Semas to the Sema domain of plexin-A1 or -A3. In addition, the polySia moiety of NRP-2 facilitates binding of CCL21 to the chemokine receptor CCR7. The intracellular signaling pathways involved in each interaction are indicated. It should be noted that the orientation of polySia between NRP-2 and CCR7 or plexin-A1/A3 is not known and may not be accurately depicted.
Although the work in this report focuses on the role of a Sema/NRP/plexin-signaling axis in DC migration, it is possible that this signaling complex also influences other DC activities (e.g., phagocytosis, antigen presentation), in which actin rearrangement is involved. In addition, each component of this signaling axis may have alternative functions by interacting with additional partners. For instance, NRPs have been shown to bind the hepatocyte growth factor [44] and galectins [45]. Regardless of their receptor partners and myriad potential functions, Semas, NRPs, and plexins are complex protein families that warrant further investigation for their significant roles in immunity, as well as in vascular, cancer, and CNS biology. These proteins represent exciting targets for therapeutic intervention in infection and inflammation and in other disease states.
AUTHORSHIP
N.M.S. conceived of the study and designed, supported, conducted, and supervised the research. S.C. designed and performed experiments. B.S.W. designed, performed, and analyzed microfluidic chamber motility studies. K.K. supported, designed, supervised, and analyzed microfluidic chamber motility studies. O.L. designed, performed, and evaluated confocal microscopy. All authors evaluated the data, and S.C. and N.M.S. wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported, in part, by the U.S. National Institutes of Health (Grant K08 HL72176-01), a grant from the Baltimore Research and Education Foundation, and institutional funds from the Institute of Human Virology (to N.M.S.) and by a grant-in-aid from the American Heart Association (to K.K.).
Glossary
- AP
alkaline phosphatase
- DC
dendritic cell
- endoN
endosialidase
- HEK
human embryonic kidney
- imDC
immature dendritic cell
- mDC
mature dendritic cell
- NRP
neuropilin
- polySia
polysialic acid
- Sema
semaphorin
- VEGF-R
vascular endothelial growth factor receptor
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Steinman R. M., Hemmi H. (2006) Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 311, 17–58. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez D., Vollmann E. H., von Andrian U. H. (2008) Mechanisms and consequences of dendritic cell migration. Immunity 29, 325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruger R. P., Aurandt J., Guan K. L. (2005) Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 6, 789–800. [DOI] [PubMed] [Google Scholar]
- 4.Yazdani U., Terman J. R. (2006) The semaphorins. Genome Biol. 7, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Wit J., Verhaagen J. (2003) Role of semaphorins in the adult nervous system. Prog. Neurobiol. 71, 249–267. [DOI] [PubMed] [Google Scholar]
- 6.Semaphorin Nomenclature Committee (1999) Unified nomenclature for the semaphorins/collapsins. Cell 97, 551–552. [DOI] [PubMed] [Google Scholar]
- 7.Giger R. J., Urquhart E. R., Gillespie S. K., Levengood D. V., Ginty D. D., Kolodkin A. L. (1998) Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron 21, 1079–1092. [DOI] [PubMed] [Google Scholar]
- 8.He Z., Tessier-Lavigne M. (1997) Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90, 739–751. [DOI] [PubMed] [Google Scholar]
- 9.Kolodkin A. L., Levengood D. V., Rowe E. G., Tai Y. T., Giger R. J., Ginty D. D. (1997) Neuropilin is a semaphorin III receptor. Cell 90, 753–762. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T., Fournier A., Nakamura F., Wang L. H., Murakami Y., Kalb R. G., Fujisawa H., Strittmatter S. M. (1999) Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99, 59–69. [DOI] [PubMed] [Google Scholar]
- 11.Tamagnone L., Artigiani S., Chen H., He Z., Ming G. I., Song H., Chedotal A., Winberg M. L., Goodman C. S., Poo M., Tessier-Lavigne M., Comoglio P. M. (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71–80. [DOI] [PubMed] [Google Scholar]
- 12.Curreli S., Arany Z., Gerardy-Schahn R., Mann D., Stamatos N. M. (2007) Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J. Biol. Chem. 282, 30346–30356. [DOI] [PubMed] [Google Scholar]
- 13.Tordjman R., Lepelletier Y., Lemarchandel V., Cambot M., Gaulard P., Hermine O., Roméo P. H. (2002) A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 3, 477–482. [DOI] [PubMed] [Google Scholar]
- 14.Bax M., van Vliet S. J., Litjens M., García-Vallejo J. J., van Kooyk Y. (2009) Interaction of polysialic acid with CCL21 regulates the migratory capacity of human dendritic cells. PLoS One 4, e6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rey-Gallardo A., Delgado-Martín C., Gerardy-Schahn R., Rodríguez-Fernández J. L., Vega M. A. (2011) Polysialic acid is required for neuropilin-2a/b-mediated control of CCL21-driven chemotaxis of mature dendritic cells and for their migration in vivo. Glycobiology 21, 655–662. [DOI] [PubMed] [Google Scholar]
- 16.Rey-Gallardo A., Escribano C., Delgado-Martín C., Rodriguez-Fernández J. L., Gerardy-Schahn R., Rutishauser U., Corbi A. L., Vega M. A. (2010) Polysialylated neuropilin-2 enhances human dendritic cell migration through the basic C-terminal region of CCL21. Glycobiology 20, 1139–1146. [DOI] [PubMed] [Google Scholar]
- 17.Kumanogoh A., Kikutani H. (2013) Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 13, 802–814. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K., Kumanogoh A., Kikutani H. (2008) Semaphorins and their receptors in immune cell interactions. Nat. Immunol. 9, 17–23. [DOI] [PubMed] [Google Scholar]
- 19.Takegahara N., Takamatsu H., Toyofuku T., Tsujimura T., Okuno T., Yukawa K., Mizui M., Yamamoto M., Prasad D. V., Suzuki K., Ishii M., Terai K., Moriya M., Nakatsuji Y., Sakoda S., Sato S., Akira S., Takeda K., Inui M., Takai T., Ikawa M., Okabe M., Kumanogoh A., Kikutani H. (2006) Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat. Cell Biol. 8, 615–622. [DOI] [PubMed] [Google Scholar]
- 20.Kumanogoh A., Suzuki K., Ch’ng E., Watanabe C., Marukawa S., Takegahara N., Ishida I., Sato T., Habu S., Yoshida K., Shi W., Kikutani H. (2002) Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J. Immunol. 169, 1175–1181. [DOI] [PubMed] [Google Scholar]
- 21.Walzer T., Galibert L., Comeau M. R., De Smedt T. (2005) Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J. Immunol. 174, 51–59. [DOI] [PubMed] [Google Scholar]
- 22.Takamatsu H., Takegahara N., Nakagawa Y., Tomura M., Taniguchi M., Friedel R. H., Rayburn H., Tessier-Lavigne M., Yoshida Y., Okuno T., Mizui M., Kang S., Nojima S., Tsujimura T., Nakatsuji Y., Katayama I., Toyofuku T., Kikutani H., Kumanogoh A. (2010) Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat. Immunol. 11, 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong A. W., Brickey W. J., Taxman D. J., van Deventer H. W., Reed W., Gao J. X., Zheng P., Liu Y., Li P., Blum J. S., McKinnon K. P., Ting J. P. (2003) CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat. Immunol. 4, 891–898. [DOI] [PubMed] [Google Scholar]
- 24.Stamatos N. M., Carubelli I., van de Vlekkert D., Bonten E. J., Papini N., Feng C., Venerando B., d’Azzo A., Cross A. S., Wang L. X., Gomatos P. J. (2010) LPS-induced cytokine production in human dendritic cells is regulated by sialidase activity. J. Leukoc. Biol. 88, 1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung W. C., Chen S. H., Paul C. D., Stroka K. M., Lo Y. C., Yang J. T., Konstantopoulos K. (2013) Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. J. Cell Biol. 202, 807–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong Z., Balzer E. M., Dallas M. R., Hung W. C., Stebe K. J., Konstantopoulos K. (2012) Chemotaxis of cell populations through confined spaces at single-cell resolution. PLoS One 7, e29211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P., Chen S. H., Hung W. C., Paul C., Zhu F., Guan P. P., Huso D. L., Kontrogianni-Konstantopoulos A., Konstantopoulos K. (2015) Fluid shear promotes chondrosarcoma cell invasion by activating matrix metalloproteinase 12 via IGF-2 and VEGF signaling pathways. Oncogene 34, 4558–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu C., Yoshida Y., Livet J., Reimert D. V., Mann F., Merte J., Henderson C. E., Jessell T. M., Kolodkin A. L., Ginty D. D. (2005) Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 307, 265–268. [DOI] [PubMed] [Google Scholar]
- 29.Chen H., Chédotal A., He Z., Goodman C. S., Tessier-Lavigne M. (1997) Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19, 547–559. [DOI] [PubMed] [Google Scholar]
- 30.Rollenhagen M., Buettner F. F., Reismann M., Jirmo A. C., Grove M., Behrens G. M., Gerardy-Schahn R., Hanisch F. G., Mühlenhoff M. (2013) Polysialic acid on neuropilin-2 is exclusively synthesized by the polysialyltransferase ST8SiaIV and attached to mucin-type O-glycans located between the b2 and c domain. J. Biol. Chem. 288, 22880–22892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamatos N. M., Zhang L., Jokilammi A., Finne J., Chen W. H., El-Maarouf A., Cross A. S., Hankey K. G. (2014) Changes in polysialic acid expression on myeloid cells during differentiation and recruitment to sites of inflammation: role in phagocytosis. Glycobiology 24, 864–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruediger T., Zimmer G., Barchmann S., Castellani V., Bagnard D., Bolz J. (2013) Integration of opposing semaphorin guidance cues in cortical axons. Cereb. Cortex 23, 604–614. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T., Nakamura F., Jin Z., Kalb R. G., Strittmatter S. M. (1998) Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat. Neurosci. 1, 487–493. [DOI] [PubMed] [Google Scholar]
- 34.Kolodkin A. L., Matthes D. J., Goodman C. S. (1993) The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75, 1389–1399. [DOI] [PubMed] [Google Scholar]
- 35.Luo Y., Raible D., Raper J. A. (1993) Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75, 217–227. [DOI] [PubMed] [Google Scholar]
- 36.Lepelletier Y., Smaniotto S., Hadj-Slimane R., Villa-Verde D. M., Nogueira A. C., Dardenne M., Hermine O., Savino W. (2007) Control of human thymocyte migration by neuropilin-1/semaphorin-3A-mediated interactions. Proc. Natl. Acad. Sci. USA 104, 5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendes-da-Cruz D. A., Brignier A. C., Asnafi V., Baleydier F., Messias C. V., Lepelletier Y., Bedjaoui N., Renand A., Smaniotto S., Canioni D., Milpied P., Balabanian K., Bousso P., Leprêtre S., Bertrand Y., Dombret H., Ifrah N., Dardenne M., Macintyre E., Savino W., Hermine O. (2014) Semaphorin 3F and neuropilin-2 control the migration of human T-cell precursors. PLoS One 9, e103405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Springer T. A. (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314. [DOI] [PubMed] [Google Scholar]
- 39.Banu N., Teichman J., Dunlap-Brown M., Villegas G., Tufro A. (2006) Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J. 20, 2150–2152. [DOI] [PubMed] [Google Scholar]
- 40.Serini G., Valdembri D., Zanivan S., Morterra G., Burkhardt C., Caccavari F., Zammataro L., Primo L., Tamagnone L., Logan M., Tessier-Lavigne M., Taniguchi M., Püschel A. W., Bussolino F. (2003) Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424, 391–397. [DOI] [PubMed] [Google Scholar]
- 41.Adams R. H., Lohrum M., Klostermann A., Betz H., Püschel A. W. (1997) The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 16, 6077–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esselens C., Malapeira J., Colomé N., Casal C., Rodríguez-Manzaneque J. C., Canals F., Arribas J. (2010) The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. J. Biol. Chem. 285, 2463–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiermaier E., Moussion C., Veldkamp C. T., Gerardy-Schahn R., de Vries I., Williams L. G., Chaffee G. R., Phillips A. J., Freiberger F., Imre R., Taleski D., Payne R. J., Braun A., Förster R., Mechtler K., Mühlenhoff M., Volkman B. F., Sixt M. (2016) Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science 351, 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulpice E., Plouët J., Bergé M., Allanic D., Tobelem G., Merkulova-Rainon T. (2008) Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 111, 2036–2045. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh S. H., Ying N. W., Wu M. H., Chiang W. F., Hsu C. L., Wong T. Y., Jin Y. T., Hong T. M., Chen Y. L. (2008) Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 27, 3746–3753. [DOI] [PubMed] [Google Scholar]