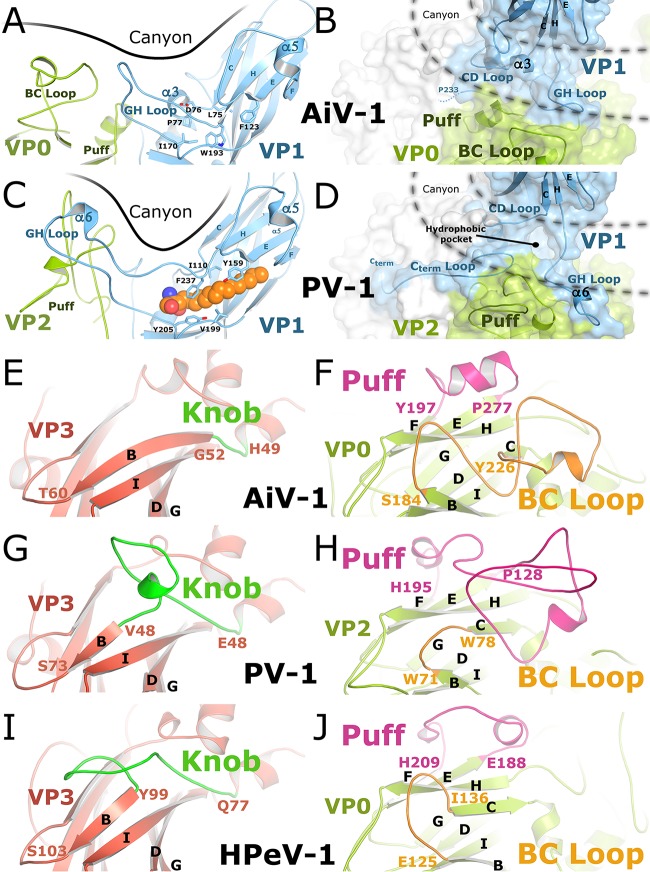
FIG 3.
Comparison of capsid surface features of AiV-1, poliovirus 1, and HPeV-1. (A to D) Side and top views of the canyons of AiV-1 (A and B) and poliovirus 1 (C and D). Capsid proteins are shown in a cartoon representation. VP1 is shown in blue, and VP2/VP0 are shown in green. A pocket factor of poliovirus 1 is shown in space-filling representation in orange. Residues that interact with the pocket factor are depicted as sticks. The molecular surface is displayed in panels B and D. (E to J) Comparison of subunits VP0 and VP3 of AiV-1 (E and F), poliovirus 1 (G and H), and HPeV-1 (I and J). VP3 of AiV-1 (E) lacks the loop knob (highlighted in green) that forms a prominent surface feature of poliovirus 1 (G) and HPeV-1 (I). The loop puff (highlighted in magenta) of AiV-1 (F) contains a single 7-residue helix and is shorter than the puffs of poliovirus 1 (H) and HPeV-1 (J). The BC loop (highlighted in orange) of AiV-1 forms a prominent feature in its capsid and is longer than those of poliovirus 1 (H) and HPeV-1 (J). Selected secondary structure elements are labeled.