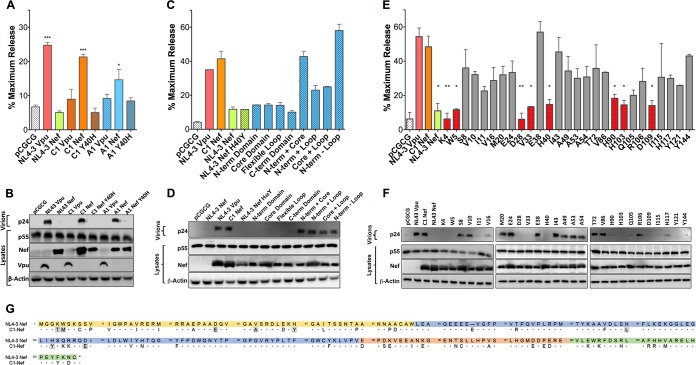
FIG 1.
Identification of residues in an HIV-1 group M Nef protein required for tetherin antagonism. (A to F) The Vpu and Nef proteins expressed by two primary HIV-1 isolates (A and B), NL4-3 Nef recombinants containing the indicated domains of C1 Nef (C and D), and C1 Nef mutants containing the indicated amino acid residues of NL4-3 Nef (E and F) were tested for their ability to rescue virus release in HEK293T cells cotransfected with HIV-1 NL4-3 Δvpu Δnef proviral DNA, a vector expressing human tetherin, and Vpu or Nef expression constructs. As controls, additional transfections were performed with constructs expressing NL4-3 Vpu, NL4-3 Nef, and an empty vector (pCGCG). At 48 h posttransfection, the accumulation of HIV-1 p24 in the cell culture supernatant was measured by an antigen capture ELISA (A, C, and E), and differences in the amounts of p24 in supernatants versus p55 Gag and Nef or Vpu in cell lysates were compared by Western blot analysis (B, D, and F). The error bars represent the standard deviations of the means for replicate assays. Differences in mean virus release compared to vector controls (A and C) or NL4-3 Vpu (E) were estimated by ordinary one-way ANOVA with adjustment for multiple comparisons (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001). (G) Amino acid sequence alignment of the HIV-1 NL4-3 and C1 Nef proteins. Dots indicate amino acid identity, dashes indicate gaps, and residues involved in tetherin antagonism are shaded. Color-coded sequences correspond to the N-terminal (yellow), globular core (blue), flexible loop (orange), and C-terminal (green) domains.