Abstract
Common and roseate terns are migratory piscivorous seabirds with major breeding colonies within feeding range of the PCB-contaminated New Bedford Harbor (NBH, MA, USA) Superfund site. Our longitudinal study shows that before PCB discharges into NBH ceased (late 1970s), tern eggs had very high but variable PCB concentrations. But egg concentrations of PCBs as well as DDE, the degradation product of the ubiquitous global contaminant DDT, have since declined. Rate constants for temporal decline of PCB congeners in tern eggs varied inversely with log10KOW (n-octanol-water partition coefficient), shifting egg congener patterns away from those characterizing NBH sediment. To estimate the toxic effects on tern eggs of PCB dioxin-like congener (DLC) exposures, we extrapolated published laboratory data on common terns to roseate terns by characterizing genetic and functional similarities in species aryl-hydrocarbon receptors (AHRs), which mediate DLC sensitivity. Our assessment of contaminant risks suggests that terns breeding near NBH were exposed historically to toxic levels of PCBs and DDE; however, acute effects on tern egg development have become less likely since the 1970s. Our approach demonstrates how comparative genetics at target loci can effectively increase the range of inference for chemical risk assessments from tested to untested and untestable species.
Keywords: Terns, Species Sensitivity, New Bedford, PCBs, Ecological Risk Assessment
TOC art
cartoon by authors using original drawings (SJ) and photos (DN).
Introduction
In 1982, New Bedford Harbor (NBH), Massachusetts, USA, was placed on the National Priorities List for cleanup under Superfund legislation due to polychlorinated biphenyl (PCB) contamination [1, 2]. NBH is a relatively large estuarine site (about 40 km long and 73 km2 area, Fig. 1) contaminated in the 1940s–1970s with discharges of PCBs, mainly Aroclors 1242 and 1016 with small quantities of Aroclor 1254 [1, 2]. PCB concentrations in NBH sediment have been reported as high as 100,000 μg g−1 [1]. Elevated concentrations of PCBs have also been reported in biota from adjoining Buzzards Bay, declining with distance from NBH [3–7]. For example, samples of the non-migratory fish, Fundulus heteroclitus, collected in 1996 along a transect from the inner harbor (Superfund site) to nearby Buzzards Bay ranged from about 300 to 3 μg g−1 [68]. Furthermore biota and environmental residues of PCBs in NBH and Buzzards Bay have unusually high proportions of less chlorinated homologs (di-, tri- and tetra-CBs) compared to those in most other areas [2, 5–8], reflecting the predominance of Aroclors 1242 and 1016 in the NBH discharges and thus providing a signature of NBH contamination.
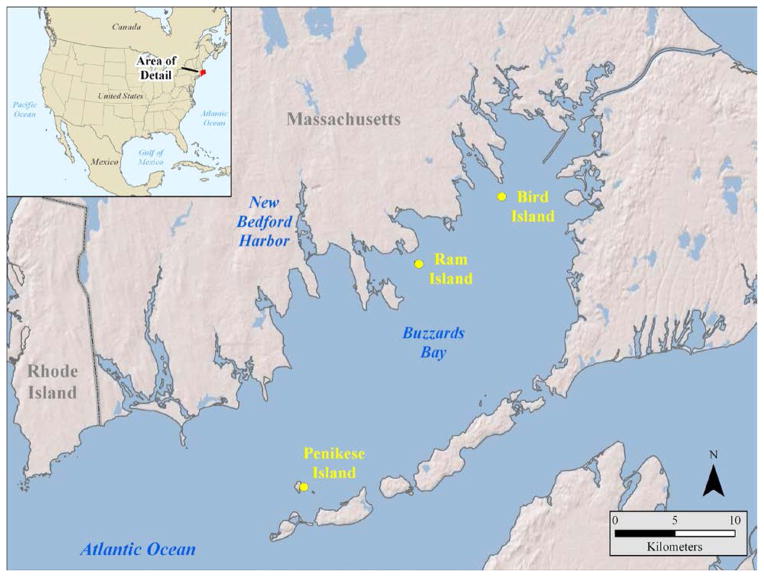
Figure 1.
Map of Buzzards Bay, Massachusetts, USA, showing the location of the PCB-contaminated site at New Bedford Harbor (NBH), and the breeding sites for common terns (Sterna hirundo) and roseate terns (S. dougallii) where eggs were collected for this study.
Common terns (CTs) Sterna hirundo and roseate terns (RTs) Sterna dougallii are small (110–140 g) piscivorous seabirds that breed at three sites in Buzzards Bay, within feeding range [9, 10] and at increasing distances from NBH: Ram Island (RI; 9 km), Bird Island (BI; 15 km) and Penikese Island (PI; 17 km) (Fig. 1). Terns of both species feed throughout the Bay, including areas adjacent to NBH, although their feeding ecologies differ: the CT feeds mainly inshore on small fish and crustaceans, whereas the RT feeds mainly in deeper water (up to 10 m) on small fish [9, 10]. Both species feed relatively infrequently within the highly contaminated area of NBH [11, 12]. However like other Buzzards Bay biota, they may be exposed to PCBs through biotic and abiotic media that have been transported out of NBH into the Bay.
Concern about effects of PCBs from NBH on terns arose because numbers of both species at BI and RI declined markedly from the 1950s to 1970s [13; see Supplemental Information, this study] and the deaths of at least seven CTs were attributed to PCB poisoning in 1970–1973 and 1989 [6; I. Nisbet, unpubl. data]. Observations that male embryos of CTs were feminized [5], and the sex-ratio of RTs was found to be skewed with an excess of females [14] raised concerns that NBH pollutants might be acting as endocrine disruptors on Buzzards Bay terns. However, feminization of male embryos may reflect a stage in normal development [3], and investigations of endocrine-disrupting effects of PCBs in CTs were inconclusive [3, 5, 14, 15].
CT eggs have been widely used in environmental monitoring [4, 10, 16] and environmental toxicology [3, 17–20], and have been found to be contaminated with PCBs at many locations in North America and Europe. In some studies, developmental toxicity observed in CTs and Forster’s terns Sterna forsteri has been ascribed to the highly toxic effects of dioxin-like PCB congeners (DLCs), i.e., those whose effects are mediated at least in part by the aryl hydrocarbon receptor (AHR) [21–27].
In this study, we use PCB contamination in Buzzards Bay CT eggs to infer population risk based on the measured sensitivity of CTs to DLCs. Furthermore, building on the recently demonstrated predictive relationship between AHR genotype and avian species differences in sensitivity to DLCs [36; 45], we leverage information on CTs to assess PCB risks to RTs (whose sensitivity to DLCs is not known currently) by comparing genetic sequence information at the AHR locus between tern species. This information is critically important for the RT, federally listed as an endangered species, since about 40% of the North American population nests in Buzzards Bay, mainly at BI and RI [9].
In addition to site-specific contamination by PCBs, concerns have also been raised about effects on terns of the global contaminant, DDT (1,1,1-trichloro-2,2-bis-(p-chlorophenyl) ethane). Residues of the DDT metabolite, ppDDE (1,1-bis-(p-chlorophenyl)-2,2-dichloroethene, referred to hereafter as DDE), are ubiquitous as a result of widespread use, and were elevated in CT eggs from Buzzards Bay in the 1970s [4; this study]. In addition, a recent study suggests (somewhat unexpectedly) that NBH may continue to act as a local source [8]. Therefore to address co-occurring contaminants of suspected importance (e.g., [69]), we included egg DDE concentrations and potential effects in our consideration of contaminant risks to Buzzards Bay tern populations.
Here, we provide a longitudinal study of PCBs and DDE in egg samples from CTs and RTs in Buzzards Bay breeding colonies from the 1970s to 2000s. We used recently collected and archived tern eggs to delineate temporal and spatial gradients in PCB congener patterns and compared them with NBH sediment cores. We used published literature to estimate the toxicity of DDE to avian species. We also used published values for the toxicity of DLCs to CT embryos, but because avian sensitivity to DLCs is known to vary widely [45; 46] we produced novel genetic and biochemical data on tern AHRs to estimate the relative sensitivity of RT and CT to DLCs. Together, this information was used to assess historical and contemporary effects of PCBs and DDE on two tern species whose breeding colonies may have been influenced by NBH estuarine Superfund site contamination. For contaminants for which there are empirical toxicity data or for which the genetic basis for toxicity is known, the approaches used here provide a model for species extrapolation, which is essential for predicting effects on untested or untestable species. In this case by combining genetic information with monitoring data for chemical contamination, we were able to infer the historical and contemporary roles that a Superfund site may have played in the major population decline of an endangered species.
Experimental details
Sample description
Between 1994 and 2005, eggs of CTs and/or RTs were collected at RI and BI (there were insufficient sample numbers to include PI in this analysis) breeding sites in Buzzards Bay (Fig. 1). Under the collection permit terms only eggs that were deserted or were incubated to term and failed to hatch were collected. Eggs were measured, weighed, and the contents were frozen in chemically-cleaned jars and held at −20 C until contaminant analysis in 2007. In addition, archived material from eggs collected in 1972 [4] was obtained from the Canadian Wildlife Service specimen bank, Ottawa, Canada (http://www.ec.gc.ca/scitech/default.asp?lang=En&n=0B9A6436-1#nwrc), representing subsamples of 5 freshly-laid eggs and 7 eggs sampled after incubation and hatching in the lab. Egg contents and chick carcasses were homogenized and processed as described earlier [4]. In total, 100 single-egg samples (43 CT, 57 RT) and 19 pools of 8–10 eggs (10 CT, 9 RT) were obtained (Table S1).
Chemical analysis
Frozen tern egg contents and archived extracts were analyzed to determine selected chemical contaminant (analyte) concentrations. Specifically, samples were analyzed using methods previously described [8], with slight modification to the analytical procedure as described more fully in SI, at the US Environmental Protection Agency, Office of Research and Development, Atlantic Ecology Division, Narragansett, RI. The 18 PCB congeners measured are those used by the National Oceanic and Atmospheric Administration National Status and Trends Program [28]. These congeners are IUPAC numbers 8, 18, 28/31, 44, 52, 66/95, 101, 105/132, 118, 128, 138, 153, 170, 180, 187, 195, 206 and 209, where PCBs 028/031, 066/095 and 105/132, which could not be distinguished in the analytical procedure, are referred to here as PCBs 028, 066 and 105. The sum of the 18 congeners in each sample is reported as Total PCBs. Concentrations of two non-ortho-substituted DLCs (IUPAC numbers 077 and 126) were measured in 26 samples, representing both species, both sites and all years. For the analysis of these DLCs, the extract was fractionated by carbon/silica column chromatography using methods in [29]. Concentrations of DDE in all samples were measured using methods described previously [8]. Representative chromatograms used for total PCBs and DDE quantification are provided in SI (Fig. S1).
For comparison to tern eggs, data on PCB congeners in two sediment cores from NBH are presented here for the first time (Table S2), and used to infer temporal profiles for NBH sediment. These two sediment cores were sampled and dated as previously described [30], and analyzed for PCBs using the same methods, facilities and analyst (SJ) as used for tern eggs here. Analytes were calculated in units of dry weight (dw), but are reported in units of adjusted wet weight (aww) as described in SI for comparison with other environmental data and with data on embryotoxicity of PCB congeners [17, 23, 27].
Statistics
Statistical analyses were conducted on analyte concentrations as dw, and analyzed using SAS version 9.2 [31] or STATISTICA version 6.0 [32]. Prior to statistical analyses, values below the MDL were assigned a value of ½MDL, and concentration data were log-transformed to equalize variance.
Data were analyzed for significant differences among species, years and locations. General Linear Models (GLMs) were used to detect differences between species and sites and to assess temporal trends, fitting data to the relationship Ln[C(t)] = k0 + k1t + k2t2 + k3*species + k4*site, where C(t) is the concentration at time t (years, where 1994 = 0), species is a binary variable for species difference (CT=1, RT=0), and site is a binary variable for site difference (RI=1, BI=0). The above equation is algebraically equivalent to C(t) = C(0)*exp(k1t + k2t2 + k3*species + k4*site), where C(0)=exp(k0). Results from these models are presented based on this form, such that group differences are presented as multiplicative factors and temporal trends as rate constants (k1 and k2). The above model was fit using both the individual egg and 19 pooled samples. Because the pooled samples are composites of multiple eggs (and are therefore analogous to an arithmetic mean across eggs), these samples would be expected to exhibit less variability than individual egg samples. Therefore to meet the regression assumption of constant variability across samples, the regression models were weighted based on the number of eggs per pool (with a weight of one for the individual egg samples). Models also were fitted for sediment data, based on the model Ln[C(t)]=k0+k1t [algebraically equivalent to C(t) = C(0)*exp(k1t)]; because of the limited number of years with sediment data, a second order k2 term could not be fitted.
Relative proportions of PCB congeners among total PCBs were calculated for each of the 100 single-egg samples, which were classified into 6 groups by species and by decade of collection (1970s, 1990s, 2000s). Proportional values were arcsine square root transformed to equalize variance prior to statistical analysis. Differences in congener patterns among sample groupings by species and period were detected using the PRIMER-E function Principal Components Analysis (PCA) [33, 34].
Molecular and biochemical characterization of the aryl hydrocarbon receptor (AHR)
RNA was isolated from livers of two RTs that were found injured and euthanized. Roseate tern AHR cDNA sequences were determined by reverse-transcription-PCR and rapid amplification of cDNA ends (RACE), and sequencing as described earlier for CT and chicken AHRs [35, 36]. The ability of in vitro-expressed RT AHRs to bind 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was assessed as described earlier [36]. Briefly, full-length AHRs from RT, CT, and chicken were synthesized by in vitro transcription and translation and their ability to bind [3H]TCDD (2 or 8 nM) was assessed using velocity sedimentation on sucrose density gradients [36].
Risk assessment for terns exposed to DLCs
To assess potential risks to terns exposed to DLCs, we used data on the embryotoxicity of PCB126 in CTs [17], World Health Organization (WHO) avian Toxic Equivalency Factors (TEFs) and assumptions of additivity [70]. Specifically, the toxicity of PCB 077, 105 and 118 were calculated as 0.5, 0.001 and 0.00001 times, respectively, the toxicity of PCB 126. Although more often calculated with reference to TCDD, here we calculated Toxic Equivalents relative to the measured in ovo effects on CTs of PCB126 (PCB126-EQs), and also expressed these values relative to the LD50 of 104 ng/g [17].
Tern breeding pair censuses
Data on the numbers of breeding pairs and productivity of common and roseate terns at the nesting sites in Buzzards Bay, MA, were compiled from various sources as described in SI.
Results and Discussion
Overall temporal decline in contamination
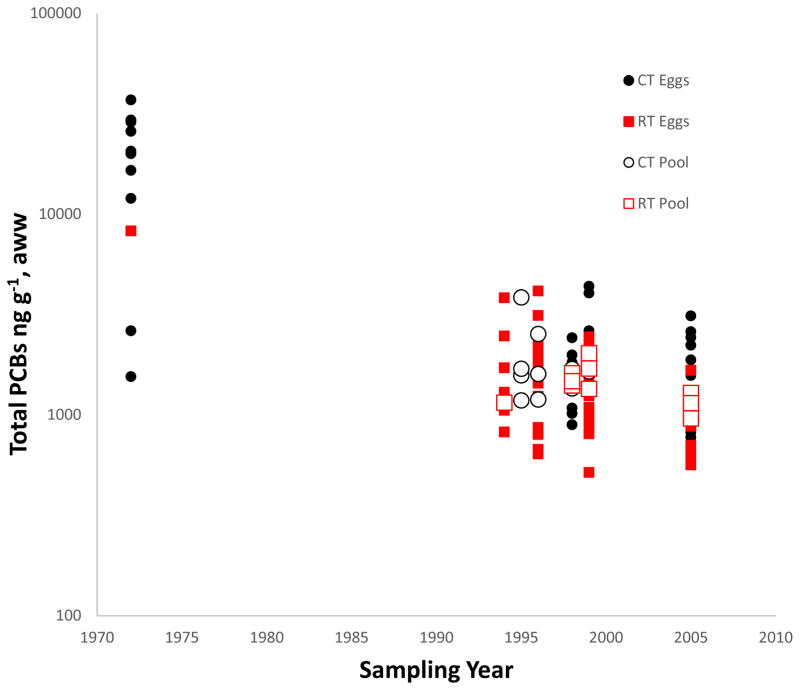
The temporal models were initially fit including the site binary variable; however because this term was not significant for any PCB congener or DDE, this term was removed from further analyses. Summary tern egg data for total PCBs and DDE show historically elevated levels (Table 1), but high variability among individuals (Fig. 2). Temporal models characterized changes, where average concentrations of total PCBs in all single egg samples declined by 87% between 1972 and 1996 (k1 = −0.051 y−1, p < 0.001; k2 = 0.002y−2, p<0.0001, Table S3) and by 90% by 2005. For comparison, total PCBs in sediment cores from NBH declined by about 73% from 1972 to 1996 (k = −0.055, p = 0.0185, Table S3).
Table 1.
Concentrations of Total PCBs and DDE, ng g−1 adjusted wet weight (aww), in eggs of common (CT) and roseate (RT) terns collected from Ram Island and Bird Island in Buzzards Bay, MA, USA. All entries are in the form (geometric mean, gmn) arithmetic mean (mn) ± SD.
| Species | Sample Type | n | Year | Total PCBs | DDE | ||||
|---|---|---|---|---|---|---|---|---|---|
| (gmn) | mn | sd | (gmn) | mn | sd | ||||
| CT | Egg | 11 | 1972 | (14853) | 20286 | 11343 | (766) | 890 | 594 |
| CT | Pool | 4 | 1995 | (1867) | 2077 | 1204 | (113) | 115 | 22 |
| CT | Pool | 3 | 1996 | (1691) | 1776 | 687 | (125) | 133 | 49 |
| CT | Egg | 8 | 1998 | (1484) | 1567 | 533 | (132) | 139 | 43 |
| CT | Pool | 2 | 1998 | (1515) | 1525 | 240 | (134) | 135 | 15 |
| CT | Egg | 8 | 1999 | (2295) | 2501 | 1146 | (115) | 120 | 37 |
| CT | Pool | 1 | 1999 | 1646 | 110 | ||||
| CT | Egg | 16 | 2005 | (1293) | 1456 | 764 | (91) | 99 | 41 |
| RT | Egg | 1 | 1972 | 8246 | 597 | ||||
| RT | Egg | 8 | 1994 | (1506) | 1700 | 997 | (82) | 89 | 37 |
| RT | Pool | 1 | 1994 | 1152 | 90 | ||||
| RT | Egg | 16 | 1996 | (1370) | 1592 | 964 | (69) | 78 | 38 |
| RT | Pool | 3 | 1998 | (1494) | 1497 | 107 | (102) | 107 | 37 |
| RT | Egg | 16 | 1999 | (1286) | 1395 | 562 | (68) | 73 | 27 |
| RT | Pool | 3 | 1999 | (1671) | 1695 | 340 | (79) | 80 | 14 |
| RT | Egg | 16 | 2005 | (948) | 985 | 278 | (40) | 45 | 21 |
| RT | Pool | 2 | 2005 | (1215) | 1217 | 102 | (73) | 75 | 14 |
Figure 2.
Concentrations (ng g−1 adjusted weight wet, aww) of Total PCBs in individual and pooled samples of common (CT) and roseate (RT) tern eggs collected in Buzzards Bay, MA, USA.
Concentrations of DDE in tern eggs declined in parallel with those of total PCBs (first order rate constants k1 = −0.052 and −0.051 y−1, respectively, for the period 1972–2005, both corresponding to half-lives of about 13 y; Table S3). The second order rate constant k2 was low for DDE compared to most PCB congeners (Table S3).
Total PCBs and DDE were highly correlated in the full data set for single eggs (r2 = 0.822, P < 0.0001), but this was in part because both were much higher in the 1972 samples than in 1994–2005 (see above). The correlation was not significant within the 1972 data set (r2 = 0.069, P = 0.84), but was highly significant within the 1994–2005 data set (r2 = 0.613, P < 0.0001). The mean ratio of total PCBs:DDE was 19.4 in 1972 and 17.9 in 1994–2005.
Previously, a compilation of data from 21 studies of CTs in 12 regions of North America showed marked decreases (by ≥ 90% in most cases) in levels of total PCBs and DDE in all areas between 1966 and 1998 [10]. However, the reported values were not rigorously comparable because analytical methods varied among studies and changed over time, with only limited inter-calibration, and because PCBs were quantitated in almost all studies by pattern-matching to Aroclor mixtures, using several different procedures. Our study confirms the decline in both total PCBs and DDE after 1972, and extends this finding by showing continued declines at about the same rates through 2005 (Table S3). It also extends temporally an earlier study in Buzzards Bay [4], including here a re-analysis of the same samples from 1972. Although we were not able to match estimates for the same individual eggs, our mean value of 20 μg g−1 total PCBs in 11 samples compares to the estimate of 29 μg g−1 total PCBs in 5 samples obtained by pattern-matching in 1972 ([4], compare [38]). However, both estimates are probably incomplete, because we measured only 18 congeners, and the earlier study did not estimate some of the less chlorinated congeners that are most characteristic of PCBs from NBH.
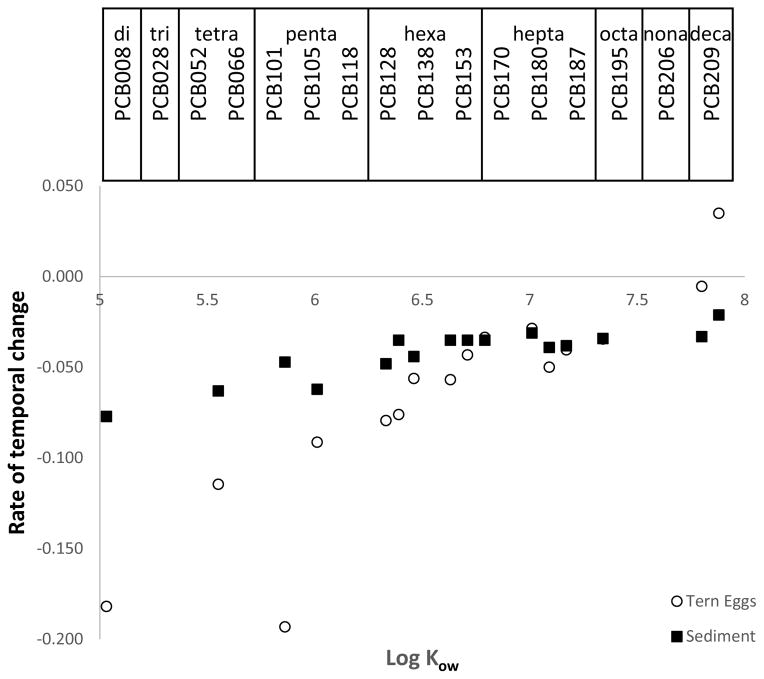
Concentrations of all individual PCB congeners except PCB209 in single and pooled tern egg samples also declined significantly during the study period, but first-order rate constants k1 decreased with increasing chlorination, from −0.193 y−1 for PCB052 to −0.005 y−1 for PCB206, with the PCB206 term not significantly different from 0 at the 95% confidence level (corresponding to half-lives of 3.6 to 131 y, respectively) (Fig. 3). First order rate constants were closely related to Kow (the partition coefficient between n-octanol and water [39, 40]) (Table S3). For the more chlorinated congeners with higher Kow (PCBs 170–206), the second order rate constants k2 were significantly positive, indicating a decelerating decline. For the less chlorinated congeners (PCBs 008–101), the second order rate constants k2 were low or negative (Table S3). For comparison, NBH sediment cores, in which total PCBs declined similarly to those in terns (Table S3), displayed a lower rate of decline in lower chlorinated congeners relative to tern eggs (Fig. 3). Because less chlorinated congeners have very short half-lives, egg concentrations of these congeners reflect what the maternal tern has been eating over a period of days prior to egg-laying, consistent with large variation observed among eggs (Table S1).
Figure 3.
Rate constants for temporal changes of PCB congeners in 2 New Bedford Harbor (NBH) sediment cores and Buzzards Bay tern eggs (Tables S2, S3) related to n-octanol-water partition coefficients (Log10 Kow) [40, 41].
Our study is one of many that have reported temporal declines in concentrations of PCBs and changes in congener patterns in a wide variety of environmental samples (reviews in [41, 42]). Several studies have reported that PCB congeners decline at different rates and that rates of decline are related to physical properties such as Kow [42]. PCB contamination at NBH was originally dominated by di-, tri- and tetra-chlorinated congeners [2, 8, 30; Table S2], resulting from the predominance of Aroclors 1242 and 1016 in the discharges. Our study shows that these congeners were selectively depleted as the PCBs were transferred from sediment to biota, and have been further depleted in the decades since the discharges ceased in 1972 (Fig. 3; Table S3). Consequently, the more recent samples of tern eggs show little of the distinctive NBH signature and resemble those from other areas remote from point sources, with a predominance of hexa-through octa-chlorinated congeners (Table S3). Our study also indicates that concentrations of the more chlorinated congeners (PCBs 170–206) in the tern eggs declined very slowly; concentrations of PCB206 increased after 1996 and those of PCB209 increased at an accelerating rate throughout the study period (Fig. 3, Tables S3). The distribution of homologues in representative examples of these Aroclors, tern eggs from two periods and NBH sediment illustrates these comparisons (Fig. S2).
After controlling for year of collection, concentrations of total PCBs and of individual congeners were higher in CT than in RT eggs for most congeners (with the ratio significantly higher than 1 at the 95% confidence level for nine congeners and total PCBs), by factors that varied with degree of chlorination and Kow, from 4.42 for PCB052 to 0.65 for PCB008, with an overall geometric mean of 1.27 (Table S3). Similarly, after controlling for year of collection, DDE concentrations in the period 1994–2005 were significantly higher in common terns than in roseate terns, by a factor of 1.46 in the egg samples.
Differences in congener proportions of total PCBs in single eggs grouped by species and period were further explored using PCA. PC1 explained 74% of total variation and documented a strong temporal trend, with high values in the 1970s and lowest values in the 2000s (Fig. S3). PC1 was most influenced by the relatively high proportions of lower chlorinated congeners (PCB ≤101) in the 1970s, and relatively high proportions of PCB105 and PCB138 in the 2000s (Fig. S3; Table S4). PC2 explained 9 % of total variation and was dominated by high values of PCB101 and low values of PCB180 (Table S4). PC2 was largely responsible for the separation of the RTs in the 2000s from the 1990s (Fig. S3).
Both CTs and RTs are exposed to PCBs primarily by ingestion of fish in Buzzards Bay up to 25 km from NBH [11, 12]. RTs have never been recorded foraging within the most contaminated zone and CTs have rarely been so recorded [12; I. Nisbet, unpubl. data]; however, variation among eggs in contaminant concentrations could be explained partly by variation in feeding areas. In a 1971–81 study, levels of organochlorine contaminants, including PCBs and DDE, in tern eggs varied widely among sampling sites and were correlated with levels in fish from the same locations [4]. Hence most of the contaminants must have been acquired by the terns in the 3–4 weeks between their return from the winter quarters and egg-laying [4, 43]. The characteristic signature of PCBs from NBH sediment, with high proportions of di-through tetra-CBs, has been observed to varying degrees in fish throughout Buzzards Bay, as well as in CTs at both BI and RI [3, 5–7]. However, differences in congener patterns between NBH sediment and biota may reflect differences in rates of degradation, losses to the atmosphere, partitioning between water and bottom or suspended sediments, uptake and retention in prey organisms, retention in the terns’ tissues, and transport into the eggs. Our finding that congener patterns changed more rapidly in the tern eggs than in the sediment core (Fig. 3) indicates that the biotic processes are important factors in congener fractionation, in addition to the physico-chemical processes affecting exposure.
Dioxin-like PCBs and molecular inferences regarding their potential effects
As for other analytes, concentrations of PCB077, PCB126, PCB105, and calculated values of PCB126-EQs (but not PCB118, Table 2) were significantly higher in 1972 than in later years (means 91.04 vs 1.95 ng g-1, respectively, for PCB126-EQs; p < 0.0001). The proportion of PCB126-EQs per total PCBs also showed a similar temporal trend (p = 0.0007), which like the trends for DLCs did not differ between species. Most of the PCB126-EQs in eggs from the 1970s were contributed by PCB077 (averaging 81%; Table 2), which remained prominent but declined in later decades (averaging 52%; Table 2). Uncertainties associated with the calculation of the toxic potency of PCBs in tern eggs are discussed in the risk assessment section.
Table 2.
Concentrations of Total PCBs and PCB congeners with dioxin-like activity in selected samples as adjusted wet weight (AWW), and calculated values of Toxicity Equivalencies of PCB126 (PCB 126 EQs) as described in Methods per references [70] and [17].
| Year | Decade | Species | AWW, ng g−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PCBs, Total | PCB77 as PCB 126 EQ | PCB105 as PCB 126 EQ | PCB118 as PCB 126 EQ | Summed PCB126 EQs | PCB126 LD50 EQs) | 100*(PCB126 EQs/Total PCBs) | |||
| 1972 | 1970 | CT | 28815 | 133.50 | 0.59 | 0.12 | 158.21 | 1.52 | 0.55 |
| 1972 | 1970 | CT | 28744 | 68.50 | 0.47 | 0.10 | 89.17 | 0.86 | 0.31 |
| 1972 | 1970 | CT | 25837 | 100.00 | 0.39 | 0.09 | 121.48 | 1.17 | 0.47 |
| 1972 | 1970 | CT | 20560 | 93.00 | 0.39 | 0.08 | 120.77 | 1.16 | 0.59 |
| 1972 | 1970 | CT | 20002 | 23.80 | 0.25 | 0.06 | 28.74 | 0.28 | 0.14 |
| 1972 | 1970 | CT | 16515 | 139.50 | 0.40 | 0.09 | 159.09 | 1.53 | 0.96 |
| 1972 | 1970 | CT | 11991 | 169.00 | 0.54 | 0.11 | 208.94 | 2.01 | 1.74 |
| 1972 | 1970 | CT | 2628 | 0.27 | 0.02 | 0.01 | 1.32 | 0.01 | 0.05 |
| 1972 | 1970 | CT | 1553 | 4.15 | 0.02 | 0.01 | 5.43 | 0.05 | 0.35 |
| 1972 | 1970 | RT | 8246 | 6.55 | 0.14 | 0.03 | 17.22 | 0.17 | 0.21 |
| 1994 | 1990 | RT | 1152 | 0.37 | 0.05 | 0.02 | 1.03 | 0.01 | 0.09 |
| 1995 | 1990 | CT | 3852 | 3.99 | 0.16 | 0.06 | 6.56 | 0.06 | 0.17 |
| 1995 | 1990 | CT | 1698 | 0.31 | 0.06 | 0.03 | 1.02 | 0.01 | 0.06 |
| 1996 | 1990 | CT | 2534 | 0.80 | 0.12 | 0.05 | 2.00 | 0.02 | 0.08 |
| 1996 | 1990 | CT | 1600 | 0.39 | 0.06 | 0.03 | 0.98 | 0.01 | 0.06 |
| 1998 | 1990 | CT | 1694 | 0.67 | 0.06 | 0.03 | 1.75 | 0.02 | 0.10 |
| 1998 | 1990 | RT | 1614 | 0.73 | 0.06 | 0.03 | 1.84 | 0.02 | 0.11 |
| 1998 | 1990 | RT | 1403 | 0.14 | 0.05 | 0.02 | 0.71 | 0.01 | 0.05 |
| 1999 | 1990 | CT | 1646 | 0.74 | 0.04 | 0.02 | 1.65 | 0.02 | 0.10 |
| 1999 | 1990 | RT | 2030 | 0.24 | 0.07 | 0.03 | 1.15 | 0.01 | 0.06 |
| 1999 | 1990 | RT | 1705 | 0.52 | 0.06 | 0.03 | 2.10 | 0.02 | 0.12 |
| 2005 | 2000 | CT | 3112 | 2.88 | 0.12 | 0.06 | 5.17 | 0.05 | 0.17 |
| 2005 | 2000 | CT | 2434 | 0.77 | 0.07 | 0.03 | 2.07 | 0.02 | 0.08 |
| 2005 | 2000 | RT | 1289 | 0.40 | 0.04 | 0.02 | 1.38 | 0.01 | 0.11 |
| 2005 | 2000 | RT | 1145 | 0.44 | 0.03 | 0.02 | 1.25 | 0.01 | 0.11 |
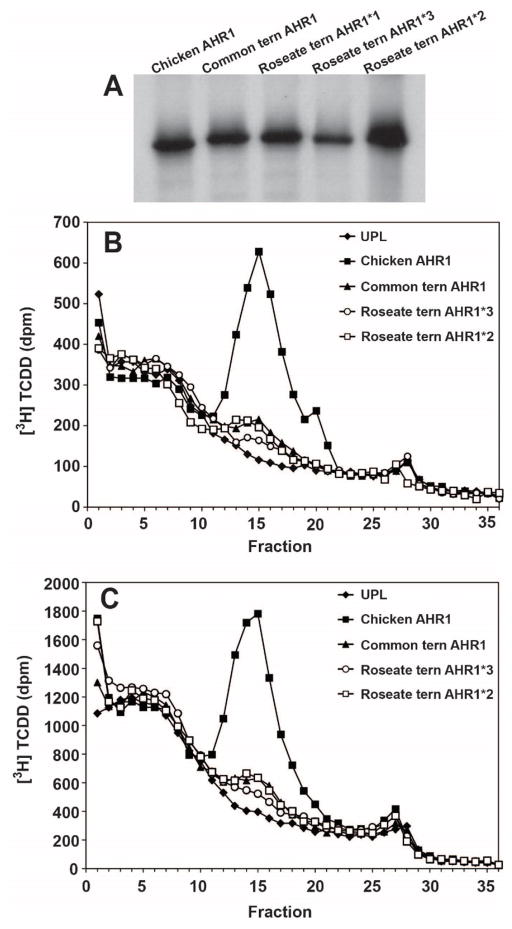
Avian species exhibit dramatic differences in sensitivity to DLCs. For example, the domestic chicken (Gallus gallus) is extremely sensitive to the effects of DLCs, whereas several other avian species, including CT, are 10- to 1,000-fold less sensitive than chickens [44, 45]. Previous studies have suggested that the amino acid sequence of the AHR1 ligand-binding domain (LBD) can be used to predict sensitivity to dioxin-like compounds [36, 44, 45]. A series of studies involving more than 85 species of birds [36, 44–46] has demonstrated that the amino acid sequence and associated biochemical properties of bird AHR1 are highly predictive of species sensitivity to DLCs, including PCBs [47]. Therefore, we inferred the sensitivity of RTs to DLCs from the genetic similarities of its AHR1 protein to that of CTs, previously demonstrated to possess a low-affinity, ‘type 3’ AHR1 [36, 45]. Full-length AHR1 cDNAs were cloned from RNA isolated from two RTs. Three allelic sequences were identified, and have been designated AHR1*1, AHR1*2, and AHR1*3. All of the RT AHR1 variants would be classified as type 3 AHRs [45]. AHR1*1 is most similar to AHR1 from the CT, with 12 synonymous nucleotide differences and no amino acid differences (Table S5; Fig. S4). AHR1*2 exhibited a single amino acid difference as compared to the CT AHR1 and RT AHR1*1. AHR1*3 was the most divergent, with 6 amino acid differences as compared to AHR1*1 and 7 differences as compared to AHR1*2 (Table S5). For comparison, these closely related CT and RT AHR1 proteins exhibit 68–74 amino acid differences as compared to the high-affinity, ‘type 1’ chicken AHR1 (Table S5).
The ligand-binding properties of the three RT AHR1 variants were compared to those of CT (low-affinity) and chicken (high-affinity) AHR1 forms by velocity sedimentation analysis using two different concentrations of radioligand ([3H]TCDD; 2 and 8 nM). All three RT AHR1 variants were indistinguishable from the CT AHR1 in their ability to bind [3H]TCDD (Fig. 4), suggesting that all three are low-affinity forms, like the CT AHR1. These results suggested that CTs and RTs are similar in sensitivity to DLCs, and allowed us to use the same risk assessment approach for both CT and RT.
Figure 4.
[3H]TCDD binding by in vitro expressed AHRs from chicken, common tern, and roseate tern. (A) In vitro transcription and translation of AHRs. AHRs were expressed in the presence of [35S]methionine. (B, C) AHRs were incubated overnight at 4°C with [3H]TCDD (B, 2 nM; C, 8 nM final concentration) and then analyzed by velocity sedimentation. Binding is measured in disintegrations per minute (dpm), where binding of [3H]TCDD to unprogrammed lysate (UPL, i.e., without AHR) measures nonspecific binding, and specific binding = total binding (radioligand binding to AHR) - nonspecific binding (radioligand binding to UPL).
Risk assessment for terns
PCB126-EQs in CT eggs sampled in 1972 (n=9) ranged from 0.01 to 2.01 times the in ovo LD50 [17] (Table 2). Thus in comparison to in ovo testing, 56% of 1972 eggs were in the lethal range (≥ 1 times LD50 ), 22% were in the range of increased deformities and reduced hatching times (≥ 0.1 but < 1 times the LD50), and 22% were below lowest tested (but toxic) concentration (≥ 0.01 but < 0.1 times the LD50) [17]. Because there was no evidence of differences between species in sensitivity to DLCs (see above), these conclusions are probably equally valid for RTs as for CTs. Furthermore, a similar summary of the toxic impact of PCB126 EQs for the tern eggs from 1990s–2000s (n=15) indicates that 87% were below lowest tested (but toxic) concentration (> 0.01 but < 0.1 times the LD50) and 13% were ≤ 0.01 times the LD50 [17]. Thus using WHO-derived TEQs and measured in ovo toxicity, the likelihood of toxic effects on tern eggs was high in the 1970s but much lower in the 1990s and 2000s. In fact because PCB concentrations in tern eggs declined at about 6% per year after 1972 (Fig. 2), risks to Buzzards Bay terns would have declined fairly rapidly.
To assess the risks of DDE, we used published data [4, 48, 49] to estimate the LC50 for embryonic death in common terns as about 3000 ng g−1 ww. Using this method, eight out of 11 of the CTs and the single RT sampled in 1972 had DDE concentrations > 0.2 times the estimated LC50, ranging up to 0.84 times the estimated LC50 (Table 1). In 1994–2005, none of the eggs of either species had DDE concentrations > 0.1 times the estimated LC50. However, the estimated value of LC50 is imprecise because only one of the references cited presented a clear comparison between DDE levels in eggs that hatched and those in eggs in which embryos died, and sample sizes in that study were very small [4].
Considered together, our risk assessments suggest that a majority of the CT eggs in 1972 would have contained concentrations of highly toxic PCB congeners but only a small proportion of CT eggs contained DDE within the range likely to cause hatching failures. Based on lower exposure, RTs would have been at slightly lower risk. However, the uncertainties associated with these estimates must also be considered. For example, other risk assessments for contaminants such as these have clearly identified the need for species-specific data for DDE [69]. In fact, the LC50 for embryotoxicity of DDE in CTs has not been well characterized: the only study in which DDE levels were compared between eggs that failed to hatch and eggs collected at random was based on a very small sample [4]. We also identify several uncertainties associated with the estimation of PCB effects. With respect to the potential for overestimation of toxic effects, (1) our calculated TEQs are driven primarily by the WHO avian TEF (van den Berg et al. 1998) of 0.5 for the ratio between the toxic potencies of PCB077 and PCB126. The basis for this value was limited when it was proposed, and subsequent evidence, including part of the study on which we base our LD50 value for PCB126 in terns [17], suggests that it may have been too high; (2) the LD50 value we use for PCB126 in terns is based on a study in which PCB126 was injected into common tern eggs [17], and this may overestimate the toxicity of PCB126 and PCB077 incorporated into eggs by natural routes in wild birds [17]. However, it should also be noted that we have no data on other potential DLCs such as TCDD or chlorinated dibenzofurans that may contribute to total EQs and are more toxic than most of the PCB congeners (e.g., van den Berg et al. 1996). This means that population risk in this study may be underestimated. Also importantly, these risk assessments are based on acute embryotoxicity and do not consider sublethal or delayed effects resulting from early life exposure or long-term exposure to lower levels of contaminants, which also could contribute to population risks.
Our data are limited to the 1970s – 2000s when egg contaminants were declining, however, it is likely that exposure of terns to DLCs and DDE, and consequent effects, would have been greater in the 1960s. For example, usage of DDT and levels of DDE in fish were much higher in the 1960s [4]. Furthermore, NBH sediment PCBs peaked in the 1970s [30], and the PCBs discharged into NBH were replaced by Aroclor 1016, with much lower levels of DLCs than the Aroclor 1242, which was used until 1971–72 [53]. Thus, based on egg contaminants, Buzzards Bay tern populations would be predicted to decline between 1950s and 1970s, followed by increases into the 1990s and 2000s. In fact, numbers of both RTs and CTs nesting at BI and RI declined rapidly between the 1950s and 1972 [13; Table S6], but have increased during the period of this study [9, 10, 50, Table S6]. While several factors including contaminant exposures [4, 10, this study] have been proposed as contributing to these changes [9, 10, 51], temporal patterns of tern egg PCB and DDE concentrations are consistent with their potential effects on Buzzards Bay tern populations (Fig. S5; Table S6). Specific to this study, declining concentrations of egg contaminants after the 1970s are concurrent with increasing tern populations (Fig. S6).
Other piscivorous birds that breed in Buzzards Bay or visit the bay in winter, including mergansers (Mergus spp.), loons (Gavia spp.), cormorants (Phalacrocorax spp.) and gulls (Laridae) may also have experienced impacts of high exposures to PCBs associated with the NBH Superfund site. Molecular genetic studies of the AHR suggest that many of these species would be similar to CTs and RTs in their relative insensitivity to DLCs, i.e., ‘type 3’ species; however, species in higher sensitivity categories could be affected by exposures up to 100 times lower than those affecting terns [45]. The approach employed here—combining analytical and molecular genetic data to perform inferential risk assessment—will have greater applicability as (1) the mechanistic basis for toxicant effects is expanded beyond the currently limited number of chemical classes, (2) functional genetic variation is extrapolated beyond a few species for which genomic information is available [57], and species ecology is further exploited to infer species vulnerabilities, even when specific chemical monitoring data of tissue concentrations are unavailable.
Supplementary Material
Acknowledgments
We thank Diana Franks (WHOI) for technical assistance. This work was supported in part by NOAA grant number NA14OAR4170074 (Woods Hole Sea Grant Project R/P-80) and by National Institute of Environmental Health Sciences (NIEHS) grant P42ES007381 (Superfund Basic Research Program at Boston University). This is tracking # ORD-015881 of the US Environmental Protection Agency, Office of Research and Development. This manuscript has been reviewed and approved for publication by the U.S. EPA. An early version of the manuscript benefitted greatly by reviews by Dr. Bryan Clark, Dr. Mark Cantwell, Dr. Barbara Bergen and Mr. Joseph LiVolsi. Approval does not signify that the contents necessarily reflect the views and policies of the U.S EPA. Mention of trade names, products, or services does not convey, and should not be interpreted as conveying official U.S. EPA approval, endorsement, or recommendation.
References
- 1.Weaver G. PCB contamination in and around New Bedford, Mass. Environ Sci Technol. 1984;18:22A–27A. doi: 10.1021/es00119a721. [DOI] [PubMed] [Google Scholar]
- 2.Bergen BT, Nelson WG, Mackay BJ, Dickerson D, Jayaraman S. Environmental monitoring of remedial dredging at the New Bedford Harbor, MA, Superfund site. Environ Monitor Assess. 2005;111:257–275. doi: 10.1007/s10661-005-8223-4. [DOI] [PubMed] [Google Scholar]
- 3.Hart CA, Nisbet ICT, Kennedy SW, Hahn ME. Gonadal feminization and halogenated environmental contaminants in common terns (Sterna hirundo): evidence that ovotestes in male embryos do not persist to the prefledgling stage. Ecotoxicol. 2003;12:125–140. doi: 10.1023/a:1022505424074. [DOI] [PubMed] [Google Scholar]
- 4.Nisbet ICT, Reynolds LM. Organochlorine residues in common terns and associated estuarine organisms, Massachusetts, USA, 1971–81. Mar Environ Res. 1984;11:33–66. [Google Scholar]
- 5.Nisbet ICT, Fry DM, Hatch JJ, Lynn B. Feminization of male common tern embryos is not correlated with exposure to specific PCB congeners. Bull Environ Contam Toxicol. 1996;57:895–901. doi: 10.1007/s001289900274. [DOI] [PubMed] [Google Scholar]
- 6.Aquatec, Inc. Unpubl report. Metcalf and Eddy; Wakefield, MA: 1990. Analytical report [PCB congeners in biota from Buzzards Bay, Massachusetts] [Google Scholar]
- 7.de Lappe BW, et al. The sampling and measurement of hydrocarbons in natural waters. In: Afghan BK, Mackay D, editors. Hydrocarbons and Halogenated Hydrocarbons in the Aquatic Environment. Plenum Press; New York: 1980. pp. 29–68. [Google Scholar]
- 8.Jayaraman S, Nacci DN, Champlin D, Pruell RJ, Rocha JK, Custer CM, Custer TW, Cantwell JM. PCBs and DDE in tree swallow (Tachycineta bicolor) eggs and nestlings from an estuarine PCB Superfund site, New Bedford Harbor, MA, USA. Environ Sci Technol. 2009;43:8387–8392. doi: 10.1021/es900255v. [DOI] [PubMed] [Google Scholar]
- 9.Nisbet ICT. Roseate tern Sterna dougallii. In: Poole A, editor. The Birds of North America Online. 370. Cornell Laboratory of Ornithology; Ithaca: NY: 2014. http://bna.birds.cornell.edu/bna/species/370. [Google Scholar]
- 10.Nisbet ICT. Common tern Sterna hirundo. In: Poole A, Gill F, editors. The Birds of North America. The Birds of North America, Inc; Philadelphia, PA: 2002. p. 618. [Google Scholar]
- 11.Tims J, Nisbet ICT, Friar MS, Mostello C, Hatch JJ. Characteristics and performance of common terns in old and newly-established colonies. Waterbirds. 2004;27:321–332. [Google Scholar]
- 12.Heinemann D. Unpubl Report to US. Fish and Wildlife Service; Newton Corner, MA: 1992. Foraging ecology of roseate terns on Bird Island, Buzzards Bay, Massachusetts. [Google Scholar]
- 13.Nisbet ICT. Terns in Massachusetts: present numbers and historical changes. Bird-Banding. 1973;44:27–55. [Google Scholar]
- 14.Nisbet ICT, Hatch JJ. Consequences of a female-biased sex ratio in a socially monogamous bird: female-female pairs in the roseate tern. Sterna dougallii Ibis. 1999;141:307–320. [Google Scholar]
- 15.French JB, Jr, Nisbet ICT, Schwabl H. Maternal steroids and contaminants in common tern eggs: a mechanism of endocrine disruption? Comp Biochem Physiol. 2001;128C:91–98. doi: 10.1016/s1532-0456(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 16.Becker PH, Thyen S, Mickstein S, Sommer U, Schmieder KR. Final Report of the Pilot Study 1996–1997. Common Wadden Sea Secretariat; Wilhelmshaven, Germany: 1998. Monitoring pollutants in coastal bird eggs in the Wadden Sea. [Google Scholar]
- 17.Hoffman DJ, Melancon MJ, Klein PN, Eisemann JD, Spann JW. Comparative developmental toxicity of planar polychlorinated biphenyl congeners in chickens, American kestrels and common terns. Environ Toxicol Chem. 1998;17:747–757. [Google Scholar]
- 18.Bosveld ATC, Nieboer R, de Bont A, Mennen J, Murk AJ, Feyk LA, Giesy JP, van den Berg M. Biochemical and developmental effects of dietary exposure to polychlorinated biphenyls 126 and 153 in common tern chicks (Sterna hirundo) Environ Toxicol Chem. 2000;19:719–730. [Google Scholar]
- 19.Lorenzen A, Shutt JL, Kennedy SW. Sensitivity of common tern (Sterna hirundo) embryo hepatocyte cultures to CYP1A induction and porphyrin accumulation by halogenated aromatic hydrocarbons and common tern egg extracts. Arch Environ Contam Toxicol. 1997;32:126–134. doi: 10.1007/s002449900164. [DOI] [PubMed] [Google Scholar]
- 20.Becker PH, Schuhmann S, Koepff C. Hatching failure in common terns (Sterna hirundo) in relation to environmental chemicals. Environ Pollut. 1993;79:207–213. doi: 10.1016/0269-7491(93)90091-2. [DOI] [PubMed] [Google Scholar]
- 21.Ankley GT, Niemi GJ, Lodge KB, Harris HJ, Beaver DL, Tillitt DE, Schwartz TR, Giesy JP, Jones PD, Hagley C. Uptake of planar polychlorinated biphenyls and 2,3,7,8-substituted polychlorinated dibenzofurans and dibenzo-p-dioxins by birds nesting in the lower Fox River and Green Bay, Wisconsin, USA. Arch Environ Contam Toxicol. 1993;24:332–344. [Google Scholar]
- 22.Senthilkumar K, Bowerman WW, Millenbah KF, Best DA, Takasuga T, Masunaga S. Polychlorinated dibenzo-p-dioxins/furans and dioxin-like biphenyls in eggs of common terns from Lime Island, St. Mary’s River, Michigan, USA. Toxicol Environ Chem. 2003;85:221–232. [Google Scholar]
- 23.Tillitt DE, Kubiak TJ, Ankley GT, Giesy JP. Dioxin-like toxic potency in Forster’s tern eggs from Green Bay, Lake Michigan, North America. Chemosphere. 1993;26:2079–2084. [Google Scholar]
- 24.Jones PD, Giesy JP, Newsted JL, Verbrugge DA, Beaver DL, Ankley GT, Tillitt DE, Lodge KB, Niemi GJ. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin equivalents in tissues of birds at Green Bay, Wisconsin, USA. Arch Environ Contam Toxicol. 1993;24:345–354. [Google Scholar]
- 25.Bosveld ATC, Gradener J, van den Berg M, Murk AJ, Brouwer A, van Kampen M, Evers EHG. Effects of PCDDs PCDFs and PCBs in common terns (Sterna hirundo) breeding in estuarine and coastal colonies in The Netherlands and Belgium. Environ Toxicol Chem. 1995;14:99–115. [Google Scholar]
- 26.Bosveld ATC, Gradener J, van Kampen M, Murk AJ, Evers EHG, van den Berg M. Occurrence and effects of PCBs, PCDDs and PCDFs in hatchlings of the common tern (Sterna hirundo) Chemosphere. 1993;27:419–427. [Google Scholar]
- 27.Bosveld ATC, van den Berg M. Effects of polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), and dibenzofurans on fish-eating birds. Environ Rev. 1995;2:147–166. [Google Scholar]
- 28.Lauenstein GG, Cantillo AY. NOAA Technical Memorandum NOS ORCA 71. National Oceanic and Atmospheric Administration; Silver Spring, MD: 1993. Sampling and Analytical Methods of the National Status and Trends Program, National Benthic Surveillance and Mussel Watch Projects. 1984–1992. Volume IV. Comprehensive Descriptions of Trace Organic Analytical Methods. [Google Scholar]
- 29.Gutjahr-Gobell DE, Black ED, Mills JL, Pruell JR, Taplin BK, Jayaraman S. Feeding the mummichog (Fundulus heteroclitus) a diet spiked with non-ortho- and mono-ortho-substituted polychlorinated biphenyls: accumulation and effects. Environ Toxicol Chem. 1999;1:699–707. [Google Scholar]
- 30.Latimer JS, Boothman WS, Pesch CE, Chmura GL, Pospelova V, Jayaraman S. Environmental stress and recovery: the geochemical record of human disturbance in New Bedford Harbor and Apponagansett Bay, Massachusetts (USA) Sci Total Environ. 2003;313:153–176. doi: 10.1016/S0048-9697(03)00269-9. [DOI] [PubMed] [Google Scholar]
- 31.SAS Institute Inc. SAS/STAT® User’s Guide, Version 9.2. 4. Vol. 1. Cary, NC: SAS Institute Inc; 1989. [Google Scholar]
- 32.StatSoft. STATISTICA™ for Windows. Version 6.0. Statsoft, Inc; Tulsa, OK: 2001. [Google Scholar]
- 33.Clarke KR, Gorley RN. PRIMER-E. Plymouth, UK: 2006. PRIMER v6: User Manual/Tutorial. [Google Scholar]
- 34.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Austr J Ecol. 1993;18:117–123. [Google Scholar]
- 35.Karchner SI, Kennedy SW, Trudeau S, Hahn ME. Towards molecular understanding of species differences in dioxin sensitivity: initial characterization of Ah receptor cDNAs in birds and an amphibian. Mar Environ Res. 2000;50:51–56. doi: 10.1016/s0141-1136(00)00045-3. [DOI] [PubMed] [Google Scholar]
- 36.Karchner SI, Franks DG, Kennedy SW, Hahn ME. The molecular basis for differential dioxin sensitivity in birds: role of the aryl hydrocarbon receptor. Proc Nat Acad Sci USA. 2006;103:6252–6257. doi: 10.1073/pnas.0509950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turle R, Norstrom RJ, Collins B. Comparison of PCB quantitation methods: Re-analysis of archived specimens of herring gull eggs from the Great Lakes. Chemosphere. 1991;22:201–213. [Google Scholar]
- 39.Hansen BG, Paya-Perez AB, Rahman M, Larsen BR. QSARs for KOW and KOC of PCB congeners: a critical examination of data, assumptions, and statistical approaches. Chemosphere. 1999;29:2209–2228. [Google Scholar]
- 40.Howard P, Meylan W, editors. Handbook of physical properties of organic chemicals. Boca Raton, FL: CRC Press, Lewis Publishers; 1997. [Google Scholar]
- 41.Loganathan BG, Kannan K. Time perspectives of organochlorine contamination in the global environment. Mar Pollut Bull. 1991;22:582–584. [Google Scholar]
- 42.Lammel G, Stemmler L. Fractionation and current time trends of PCB congeners: evolvement of distributions 1950–2010 studied using a global atmosphere-ocean general circulation model. Atmos Chem Phys. 2012;12:7199–7213. [Google Scholar]
- 43.Moore DJ, Williams TD, Morris RD. Mate-provisioning, nutritional requirements for egg-production, and primary reproductive effort of female common terns. Sterna hirundo J Avian Biol. 2000;31:183–196. [Google Scholar]
- 44.Head JA, Hahn ME, Kennedy SW. Key amino acids in the aryl hydrocarbon receptor predict dioxin sensitivity in avian species. Environ Sci Technol. 2008;42:7535–7541. doi: 10.1021/es801082a. [DOI] [PubMed] [Google Scholar]
- 45.Farmahin R, Manning GE, Crump D, Wu D, Mundy LJ, Jones SP, Hahn ME, Karchner SI, Giesy JP, Bursian SJ, Zwiernik MJ, Fredricks TB, Kennedy SW. Amino acid sequence of the ligand binding domain of the aryl hydrocarbon receptor 1 (AHR1) predicts sensitivity of wild birds to effects of dioxin-like compounds. Toxicol Sci. 2013;131:139–152. doi: 10.1093/toxsci/kfs259. [DOI] [PubMed] [Google Scholar]
- 46.Farmahin R, Jones SP, Crump D, Hahn ME, Giesy JP, Zwiernik MJ, Bursian SJ, Kennedy SW. Species-specific relative AHR1 binding affinities of 2,3,4,7,8-pentachlorodibenzofuran explain avian species differences in its relative potency. Comp Biochem Physiol C Toxicol Pharmacol. 2014;161:21–25. doi: 10.1016/j.cbpc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Manning GE, Farmahin R, Crump D, Jones SP, Klein J, Konstantinov A, Potter D, Kennedy SW. A luciferase reporter gene assay and aryl hydrocarbon receptor 1 genotype predict the LD50 of polychlorinated biphenyls in avian species. Toxicol Appl Pharmacol. 2012;263:390–401. doi: 10.1016/j.taap.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Fox GA. Eggshell quality: its ecological and physiological significance in a DDE-contaminated common tern population. Wilson Bull. 1976;88:459–477. [Google Scholar]
- 49.Switzer B, Lewin V, Wolfe FH. DDE and reproductive success in some Alberta common terns. Can J Zool. 1973;51:1081–1086. doi: 10.1139/z73-157. [DOI] [PubMed] [Google Scholar]
- 50.Breton AR, Nisbet ICT, Mostello CS, Hatch JJ. Age-dependent survival and breeding dispersal within a metapopulation of common terns. Sterna hirundo Ibis. 2014;156:534–547. [Google Scholar]
- 51.U.S. Fish and Wildlife Service. Caribbean Roseate Tern and North Atlantic Roseate Tern (Sterna dougallii dougallii). 5-Year Review: Summary and Evaluation. U.S. Fish and Wildlife Service, Boquerón, Puerto Rico, and Concord; New Hampshire: 2010. [Google Scholar]
- 52.Nisbet ICT, Drury WH. Measuring breeding success in common and roseate terns. Bird-Banding. 1972;43:97–106. [Google Scholar]
- 53.Weaver G. PCB contamination in and around New Bedford. Mass Environ Sci Technol. 1984;18:22A–7A. doi: 10.1021/es00119a721. [DOI] [PubMed] [Google Scholar]
- 54.Hatch JJ. Double-crested cormorant Phalacrocorax auritus. In: Poole A, Gill F, editors. The Birds of North America. 441 The Birds of North America, Inc; Philadelphia, PA: 1999. [Google Scholar]
- 55.Gilbertson N, Kubiak T, Ludwig J, Fox G. Great lakes embryo mortality, edema, and deformities syndrome (GLEMEDS) in colonial fish-eating birds: Similarity to chick-edema disease. J Toxicol Environ Health. 1991;33:455–502. doi: 10.1080/15287399109531538. [DOI] [PubMed] [Google Scholar]
- 56.Wires L. The Double-crested Cormorant: Plight of a Feathered Pariah. New Haven: Yale University Press; 2013. [Google Scholar]
- 57.LaLone CA, Villeneuve DL, Burgoon LD, Russom CL, Helgen HW, Berninger JP, Tietge JE, Severson MN, Cavallin JE, Ankley GT. Molecular target sequence similarity as a basis for species extrapolation to assess the ecological risk of chemicals with known modes of action. Aquat Toxicol. 2013;144–145:141–154. doi: 10.1016/j.aquatox.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Wise SA, Poster DL, Schantz MM, Kucklick JR, Sander LC, Lopez de Alda MJ, Schubert P, Parris RM, Porter BJ. Three new mussel tissue standard reference materials (SRMs) for the determination of organic contaminants. Anal Bioanal Chem. 2004;378:1213–1231. doi: 10.1007/s00216-003-2401-4. [DOI] [PubMed] [Google Scholar]
- 59.Nisbet ICT. Dependence of fledging success on egg-size, parental performance and egg-composition among common and roseate terns, Sterna hirundo and S. dougallii. Ibis. 1978;110:205–214. [Google Scholar]
- 60.Ar A, Rahn H. Water in the avian egg: overall budget of incubation. Amer Zool. 1980;20:477–484. [Google Scholar]
- 61.Nisbet ICT. Population models for common terns in Massachusetts. Bird-Banding. 1978;49:50–58. [Google Scholar]
- 62.Nisbet ICT. Status and trends of the Roseate Tern Sterna dougallii in North America and the Caribbean. Report to U.S. Fish & Wildlife Service, Office of Endangered Species; Newton Corner, Mass: 1980. [Google Scholar]
- 63.Nisbet ICT. Report to US. Fish & Wildlife Service, Office of Endangered Species; Newton Corner, Mass: 1981. Biological characteristics of the Roseate Tern Sterna dougallii. [Google Scholar]
- 64.Burger J, Nisbet ICT, Gochfeld M. Temporal patterns in reproductive success of the endangered Roseate Tern (Sterna dougallii) nestingBird Island, Massachusetts, and Cedar Beach, New York. Auk. 1996;113:131–142. [Google Scholar]
- 65.Austin OL. Site tenacity, a behaviour trait of the common tern (Sterna hirundo Linn) Bird-banding. 1949;20:1–39. [Google Scholar]
- 66.Austin OL. Group adherence in the Common Tern. Bird-Banding. 1951;22:1–15. [Google Scholar]
- 67.Austin OL. The status of the Cape Cod terns in 1944: A behaviour study. Bird-Banding. 1945;16:10–27. [Google Scholar]
- 68.Nelson WG, Bergen BJ, Benyi SJ, Morrison G, Voyer RA, Strobel CJ, Rego S, Thursby G, Pesch CE. New Bedford Harbor long-term monitoring: baseline sampling. U.S. Environmental Protection Agency, National Health and Environmental Effects Research Laboratory, Atlantic Ecology Division; Narragansett, RI: 1996. EPA/600/R–96/097. [Google Scholar]
- 69.Strause KD, Zwiernik MJ, Im SH, Bradley PW, Moseley PP, Kay DP, Park CS, Jones PD, Blankenship AL, Newsted JL, Giesy JP. Risk assessment of great horned owls (Bubo virginianus) exposed to polychlorinated biphenyls DDT along Kalamazoo River, Michigan USA. Environmental Toxicology and Chemistry. 2007;26:1386–1398. doi: 10.1897/06-382r.1. [DOI] [PubMed] [Google Scholar]
- 70.van den Berg M, Birnbaum L, Bosveld ATC, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Haegawa R, Kennedy SW, Kubiak T, Larsen JC, Van Leeuwen FXR, Liem AKD, Nolt C, Petersen RE, Poellinger L, Safe S, Schrenk D, Tillit D, Tysklind M, Younes M, Waern F, Zacharewski T. Toxic equivalency Factors (TEFs) for PCBs, PCDDs, PCDFs for human wildlife. Environmental Health Perspectives. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.