Abstract
Background and purpose
PU-H71 is a purine-scaffold Hsp90 inhibitor developed to overcome limitations of conventional Hsp90 inhibitors. This study was designed to investigate the combined effect of PU-H71 and heavy ion irradiation on human tumor and normal cells.
Materials and methods
The effects of PU-H71 were determined by monitoring cell survival by colony formation, and DNA double-strand break (DSB) repair by γ-H2AX foci and immuno-blotting DSB repair proteins. The mode of cell death was evaluated by sub-G1 DNA content (as an indicator for apoptosis), and mitotic catastrophe.
Results
PU-H71 enhanced heavy ion irradiation-induced cell death in three human cancer cell lines, but the drug did not radiosensitize normal human fibroblasts. In irradiated tumor cells, PU-H71 increased the persistence of γ-H2AX foci, and it reduced RAD51 foci and phosphorylated DNA-PKcs, key DSB repair proteins involved in homologous recombination (HR) and non-homologous end joining (NHEJ). In some tumor cell lines, PU-H71 altered the sub-G1 cell fraction and mitotic catastrophe following carbon ion irradiation.
Conclusion
Our results demonstrate that PU-H71 sensitizes human cancer cells to heavy ion irradiation by inhibiting both HR and NHEJ DSB repair pathways. PU-H71 holds promise as a radiosensitizer for enhancing the efficacy of heavy ion radiotherapy.
Keywords: PU-H71, Hsp90, Heavy ion radiotherapy, Radiosensitization, DNA repair
Heat shock protein 90 (Hsp90) plays an important role in stabilizing and regulating many proteins associated with the development and progression of malignant tumors [1]. In addition, Hsp90 can regulate the function of several of its client proteins involved in radioresistance [2,3]; hence, Hsp90 inhibition enhances radiosensitivity of tumor cells and therefore holds promise as an adjunct to cancer radiotherapy. Several Hsp90 inhibitors have been reported to radiosensitize tumor cells in vitro and in vivo. For example, geldanamycin and its derivatives enhance radiation-induced cell death of various human tumors via multiple mechanisms: inhibition of DNA repair and pro-survival signaling pathways; enhanced apoptosis; and impairment of cell cycle checkpoint function [4–7]. However, certain well-studied Hsp90 inhibitors have properties that significantly limit their therapeutic usefulness, such as hepatotoxicity, metabolic instability, poor aqueous solubility, and poor bioavailability [8–10].
In order to overcome these limitations, the Chiosis laboratory designed novel purine scaffold Hsp90 inhibitors. Among these, PU-H71 is potent, with nanomolar concentrations shown to bind Hsp90, cause degradation of the representative Hsp90 client protein Her2, and inhibit growth of the SKBr3 breast cancer cell line [11]. Interestingly, PU-H71 has higher binding affinity to Hsp90 complexes from cancer cells than those in normal tissue, so this drug confers selective cytotoxicity toward cancer cells. Indeed, PU-H71 is a potent growth inhibitor, and it induces apoptosis in various cancer cell lines [12,13]. Owing to these desirable properties, PU-H71 is an attractive agent for cancer therapy.
Heavy ion radiotherapy has several advantages over conventional photon radiotherapy. It is more effective in tumor cell killing than photon radiotherapy, it shows a lower oxygen-enhancement ratio (important for hypoxic tumor control), and it can be targeted to tumors more accurately which improves normal tissue sparing [14,15]. Given these benefits of heavy ion radiotherapy, it is important to seek strategies to further enhance its efficacy. DNA double-strand breaks (DSBs) are the critical lesion induced by ionizing radiation, and the failure to repair DSBs can result in cell death. Major mechanisms for DSB repair are homologous recombination (HR) and non-homologous end joining (NHEJ), and disturbing these DSB repair machineries sensitizes cells to the cytotoxic effects on ionizing radiation.
PU-H71 was shown to enhance cell death in X-irradiated SQ5 and A549 lung cancer cells by inhibiting HR repair [16]. However, the effect of PU-H71 in heavy ion irradiated cells has not been previously investigated. Here we tested the effects of PU-H71 on cellular radiosensitivity to heavy ion irradiation. There are contrasting views on the relative importance of NHEJ and HR DSB repair pathways in cells irradiated with high LET heavy ions. According to Okayasu et al. [17] and Wang et al. [18], high LET irradiation creates DNA damage that is not efficiently repaired by NHEJ. In addition, high LET radiation-induced complex DNA damage efficiently promotes DNA end resection, a crucial step for HR [19]. Moreover, Gerelchuluun et al. [20] showed that NHEJ is important for cell survival after proton or carbon irradiation, but that the HR pathway was even more important after carbon ion irradiation. These findings suggest that inhibition of the HR pathway will efficiently sensitize cancer cells to high LET heavy ion irradiation. In contrast, Takahashi et al. [21] found that inhibition of NHEJ had a greater radiosensitizing effect than inhibition of HR to high LET radiation. In order to gain insight into mechanisms of PU-H71 induced radiosensitizing effects, the present study focused on its effects on both NHEJ and HR DSB repair pathways.
Materials and methods
Cell lines and cell culture
Human lung adenocarcinoma cell lines (A549 and H1299) and normal human lung fibroblasts (HFL-III) were obtained from RIKEN BioResource Center (RIKEN, Tsukuba, Japan) and the American Type Culture Collection (Maryland, USA). HeLa-SQ5 was obtained from RIKEN: The cell line originally called SQ5 was identified by RIKEN as a HeLa derivative by Short Tandem Repeat profiling in 2015 (performed twice), so here we designate this cell line as HeLa-SQ5. HeLa-SQ5, A549 and HFL-III were cultured in alpha-MEM medium (Wako Chemical, Osaka, Japan) supplemented with 10% FBS (HeLa-SQ5 and A549) or 15% FBS (HFL-III). H1299 was cultured in RPMI-1640 medium (Wako Chemical) supplemented with 10% FBS. Cells were grown in a humidified incubator with 5% CO2 at 37 °C.
Drug treatment and irradiation
PU-H71 was purchased from Tocris Bioscience (Bristol, UK), and dissolved in dimethyl sulfoxide (DMSO). Exponentially growing cells were incubated with PU-H71 (1 µM or DMSO for 24 h, then cells were vertically irradiated with 290 MeV/n carbon ions [6 cm Spread-Out Bragg Peak (SOBP), LET: approximately 50 keV/µm] with the Heavy Ion Medical Accelerator in Chiba (HIMAC) at the National Institute of Radiological Sciences (NIRS). For X-irradiation, cells were irradiated using a X-ray generator (TITAN-320, Shimadzu, Japan) operated at 200 kV, 200 mA. After irradiation, medium was replaced with fresh drug-free medium.
Colony formation assay
Cells were seeded at appropriate concentrations in 6 cm dishes and then treated with drug (DMSO or 1 µM PU-H71) and/or heavy ions. After 10–14 days of incubation, colonies were fixed and stained with 0.2% crystal violet. Colonies containing at least 50 cells were counted, and the plating efficiency of each conditioned group was calculated as 0.21 for HeLa-SQ5 cells, 0.25 for H1299 cells and 0.95 for A549 cells. The surviving fraction was normalized based on the plating efficiency determined from corresponding controls (DMSO- or PU-H71-treated cells). Experiments were repeated two or three times.
Immunofluorescence measurements
Cells were grown on 4-well chamber slides (Nunc, Rochester, NY), fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, and blocked in 3% BSA. For γ-H2AX and RAD51 foci measurements, the following primary and secondary antibodies were used: anti-phospho-Histone H2AX (Ser139) (Millipore, Billerica, MA), anti-RAD51 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Cyclin B (Millipore), Alexa 488-anti-mouse secondary antibody and Alexa 594-anti-rabbit secondary antibody (Thermo Fisher Scientific, Rockford, IL). The slides were covered with cover glasses with ProLong Gold antifade agent with DAPI (Life Technologies, Grand Island, NY). Fluorescence images were captured using an Olympus BX51 fluorescence microscope. The number of γ-H2AX or RAD51 foci was counted in >50 nuclei per experiment. Experiments were repeated two or three times.
Western blotting
Whole cell lysates were loaded onto SDS–PAGE gels, and transferred onto PVDF or nitrocellulose membranes. The membranes were blocked with 0.2% I-Block (Tropix, Bedford, MA) and then incubated with the following primary antibodies overnight at 4 °C: GAPDH (Cell Signaling Technology, Dancers, MA), RAD51 (Santa Cruz Biotechnology, CA), EGFR (Abcam, Cambridge, MA), phospho ERK1/2 (Cell Signaling Technology), phospho DNA-PKcs (S2056 and T2609, Abcam), Ku70 (Thermo Fisher Scientific), and Ku80 (Thermo Fisher Scientific). Membranes were washed with TBST, and incubated with anti-rabbit or anti-mouse secondary antibody (Sigma–Aldrich, St. Louis, MO). Protein expression levels were quantified using an ImageQuant LAS-4000 system (Fuji Film, Tokyo, Japan).
Measurements of sub-G1 cell populations
Cells were fixed in 70% cold ethanol, and stained with 50 µg/ml propidium iodide (Sigma–Aldrich) in the presence of RNase (Wako Chemical). Cellular DNA content was measured with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA), and at least 10,000 cells were analyzed per condition. Data were evaluated with Cell-QuestPro Software (BD Biosciences) and ModFit Software (Verity Software House, Topsham, ME).
Evaluation of mitotic catastrophe
Cells were determined to undergo mitotic catastrophe when cells had nuclei with more than two distinct lobes [22]. Cells were grown on 4-well chamber slides (Nunc), irradiated, and 72 h later cells were fixed in 4% paraformaldehyde for 15 min at 4 °C, and washed in PBS. The slides were covered with cover glasses with ProLong Gold antifade agent with DAPI. At least 300 cells were analyzed per condition using an Olympus BX51 fluorescence microscope, and the percentages of cells undergoing mitotic catastrophe were calculated.
Statistical analyses
Statistical analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC). Student’s t-tests were used to compare between PU-H71 treated cells and non-treated cells, and two sided p < 0.05 was considered statistically significant.
Results
PU-H71 sensitizes cancer cells to heavy ion irradiation
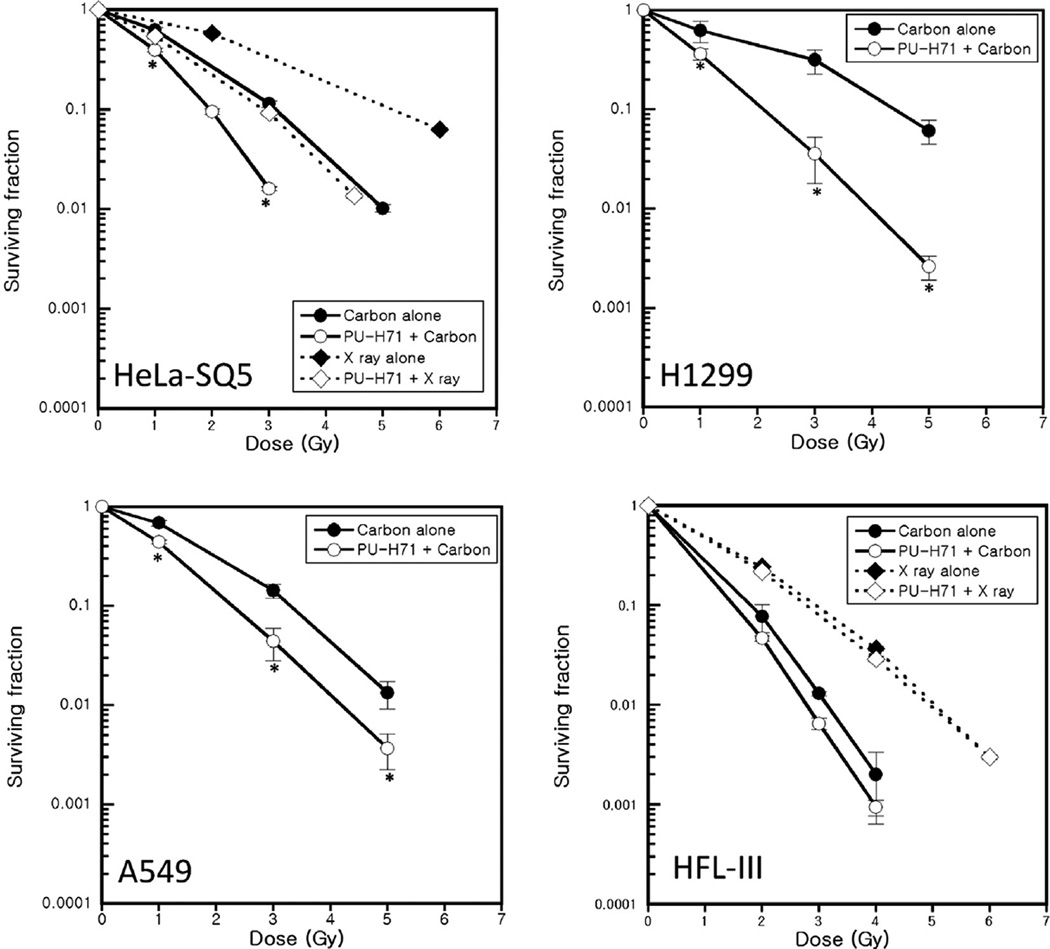
We used colony formation assays to determine the cytotoxic effects of PU-H71 in heavy ion irradiated human cancer and normal cell lines. For comparison, we also measured surviving fraction in X irradiated HeLa-SQ5 cells and HFL-III cells, and we observed the radiosensitizing effect of PU-H71 (Fig. 1). As observed in X-irradiated HeLa-SQ5 cells, all three tumor cell lines showed a significant increase in 290 MeV/n carbon ion-induced cell death when pre-treated with 1 µMPU-H71 (Fig. 1). The radiosensitivity enhancement ratios measured at a survival rate of 10% were 1.59, 1.38, and 2.05 for HeLa-SQ5, A549, and H1299 cells, respectively. In contrast, PU-H71 did not significantly enhance sensitivity of normal human fibroblasts to carbon ion irradiation or to X-irradiation. We also assessed the radiosensitizing effects of PU-H71 in 200 keV/µm iron ion irradiated tumor cell lines. As with carbon ions, PU-H71 sensitized cancer cells to iron ion radiation (data not shown). These results clearly demonstrate that PU-H71 sensitizes various cancer cell lines to high LET heavy ion radiation and to low LET X-rays.
Fig. 1.
PU-H71 sensitizes human cancer cells to heavy ion irradiation. HeLa-SQ5, H1299, A549 and HFL-III cells were pretreated with 1 µM PU-H71 or DMSO for 24 h, and irradiated with carbon ions (LET; ~50 keV/µm) or X rays. The ability of PU-H71 to radiosensitize human tumor and normal cells was determined by colony formation assay. Colonies containing more than 50 cells were counted as surviving cells. Data represent mean ± SEM for two or three determinations. * indicates p < 0.05.
PU-H71 inhibits DSB repair in carbon-irradiated cancer cells
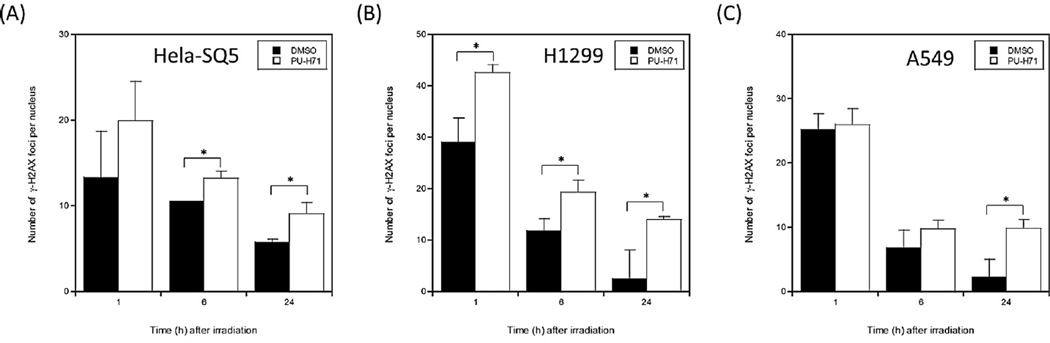
To gain insight into the mechanism(s) by which PU-H71 sensitizes cancer cells to carbon ion radiation, we measured the number of γ-H2AX foci initially induced by carbon ion irradiation, and the decrease in these foci over time, reflecting DSB repair. As shown in Fig. 2 and Supplementary Fig. 1, the number of γ-H2AX foci 1 h after carbon ion irradiation was similar or somewhat higher in PU-H71 treated cells, but 24 h after irradiation, PU-H71 treated cells had significantly more γ-H2AX foci than controls in all three cancer cell lines tested. The persistent γ-H2AX foci with PU-H71 treatment indicates that this drug inhibits repair of carbon ion radiation-induced DSBs.
Fig. 2.
PU-H71 inhibits DSB repair in carbon irradiated cancer cells. HeLa-SQ5 (A), H1299 (B), and A549 (C) cells were fixed at various times (1, 6 or 24 h) after 2 Gy carbon ion irradiation. The cells were stained using anti-γ-H2AX (Ser139) antibodies with DAPI as nuclear counter stain. The number of γ-H2AX foci was counted in >50 nuclei per experiment. All the data in this figure were obtained by subtracting the number of γ-H2AX foci in non-irradiated control values (PU-H71 treated or mock treated group). Data represent mean ± SEM for two or three determinations. * indicates p < 0.05.
PU-H71 inhibits HR and NHEJ repair pathways in carbon ion irradiated cancer cells
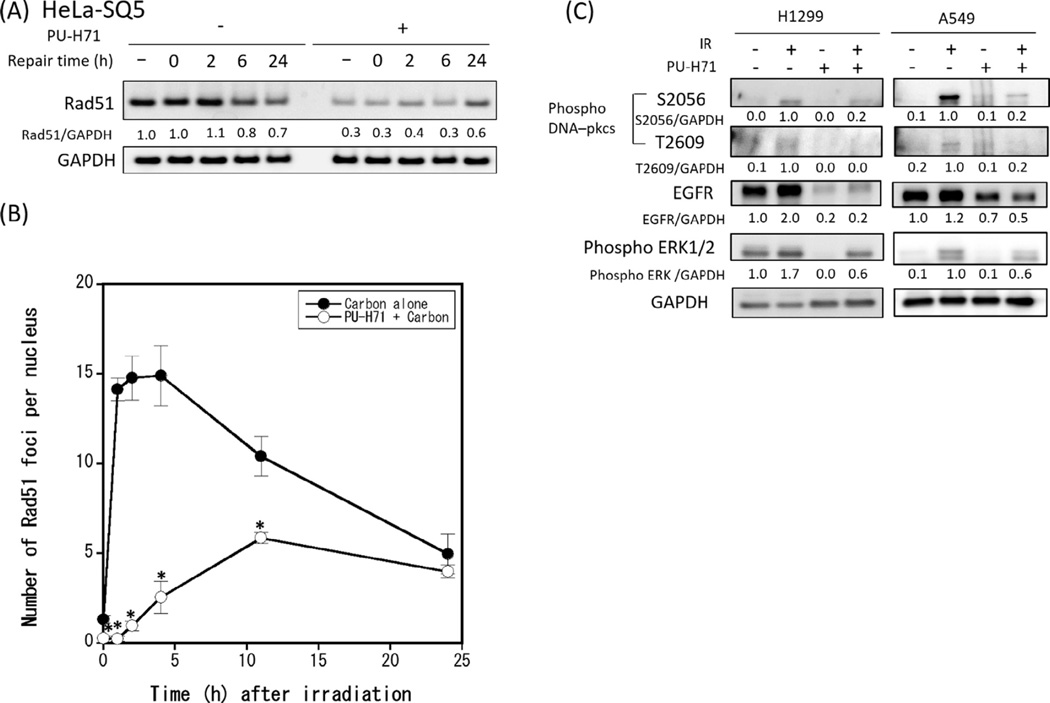
We investigated the mechanism by which PU-H71 affects DSB repair by examining critical components of HR and NHEJ that together comprise the main DSB repair pathways. We first assessed expression levels of RAD51, a key protein in the HR pathway, in carbon irradiated cancer cells with or without PU-H71. PU-H71 treatment decreased RAD51 protein in all three cell lines tested (Fig. 3A and Supplementary Fig. 2), and PU-H71 had a similar effect on RAD51 protein in X-irradiated HeLa-SQ5 cells (Supplementary Fig. 3). We also examined the kinetics of RAD51 foci formation after carbon ion irradiation in HeLa-SQ5 cells. As shown in Fig. 3B and Supplementary Fig. 4, the number of carbon ion radiation-induced RAD51 foci was nearly maximal within 2 h after irradiation in the absence of PU-H71, whereas PU-H71 significantly delayed RAD51 focus formation, with maximal levels reached 10 h after irradiation. PU-H71 also reduced the number of foci per cell to <50% of non-drug treated controls. These results indicate that PU-H71 inhibits the recruitment (and/or retention) of RAD51 to DSBs and hence inhibits DSB repair by RAD51-mediated HR.
Fig. 3.
PU-H71 affects the HR and NHEJ repair pathway in human cancer cells. (A) HeLa-SQ5 cells were pretreated with 1 µMPU-H71 for 24 h, and irradiated with 4 Gy carbon ions. At indicated times after irradiation, cells were collected and total cellular proteins were immunoblotted with RAD51 and GAPDH antibodies. GAPDH was used as loading control. Numbers below the bands correspond to the fold changes normalized to non-drug treated and non-irradiated control cells. (B) HeLa-SQ5 cells pretreated with PU-H71 (1 µM) or DMSO were fixed at indicated times after 2 Gy carbon ion irradiation. Cells were incubated with anti-RAD51 and anti-Cyclin B, and the number of RAD51 foci per nucleus was counted in Cyclin-B stained cells. Data represent mean ± SEM of three independent experiments. * indicates p < 0.05. (C) To assess the effects of PU-H71 on DNA-PKcs activation we collected A549 cells and H1299 cells 2 h after 2 Gy carbon ion irradiation and total cellular proteins were immunoblotted with antibodies against phosphorylated DNA-PKcs (phospho S2056 and phospho T2609), EGFR, phosphorylated ERK, and GAPDH. GAPDH was used as loading control.
We next tested whether PU-H71 affects the expression of Ku70 or Ku80, two proteins with key early roles in NHEJ, binding DNA ends at DSBs. As shown in Supplementary Fig. 5, neither Ku70 nor Ku80 protein levels were affected by PU-H71 treatment in three cancer cell lines. At a later stage in the NHEJ pathway, the DNA-PK catalytic subunit (DNA-PKcs) is phosphorylated on several residues including S2056 and T2609; these phosphorylation events are important for proper DNA-PKcs activation and efficient completion of NHEJ [23–25]. Interestingly, PU-H71 treatment inhibited DNA-PKcs S2056 and T2609 phosphorylation in both H1299 and A549 cancer cells (Fig. 3C), indicating that PU-H71 inhibits DNA-PKcs activation in response to carbon irradiation. We also monitored levels of EGFR, and phospho-ERK1/2 because these proteins are known to affect the activity of RAD51 and DNA-PKcs [26–28]. As shown in Fig. 3C, PU-H71 decreased expression of both EGFR and phospho-ERK1/2, thus these changes may underlie the reduced RAD51 expression and reduced DNA-PKcs activation. Together, the results demonstrate that PU-H71 treatment disrupts DSB repair by both NHEJ and HR repair pathways in carbon ion irradiated cancer cells, accounting for the marked sensitization by PU-H71 of cancer cells to heavy ion radiation.
PU-H71 alters cell death pathways depending on cell type
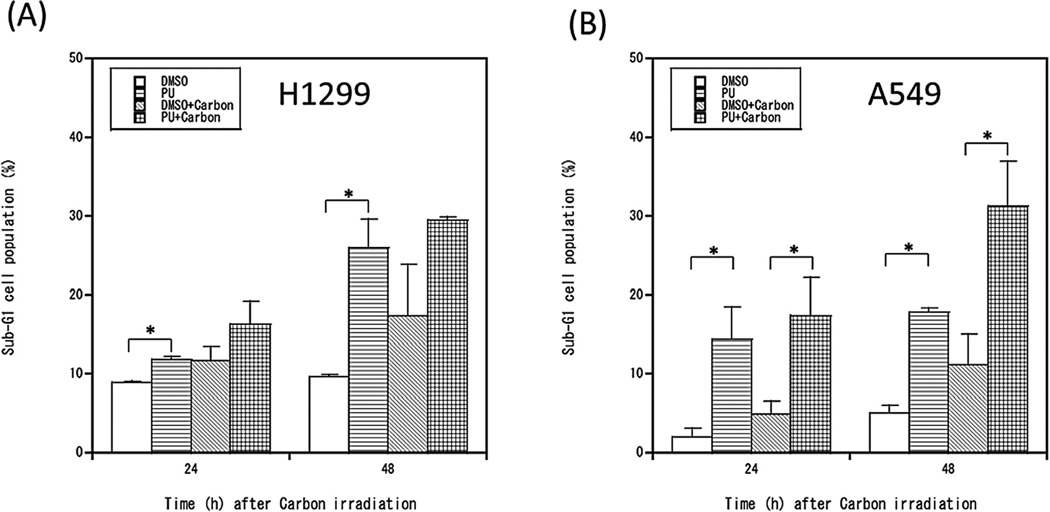
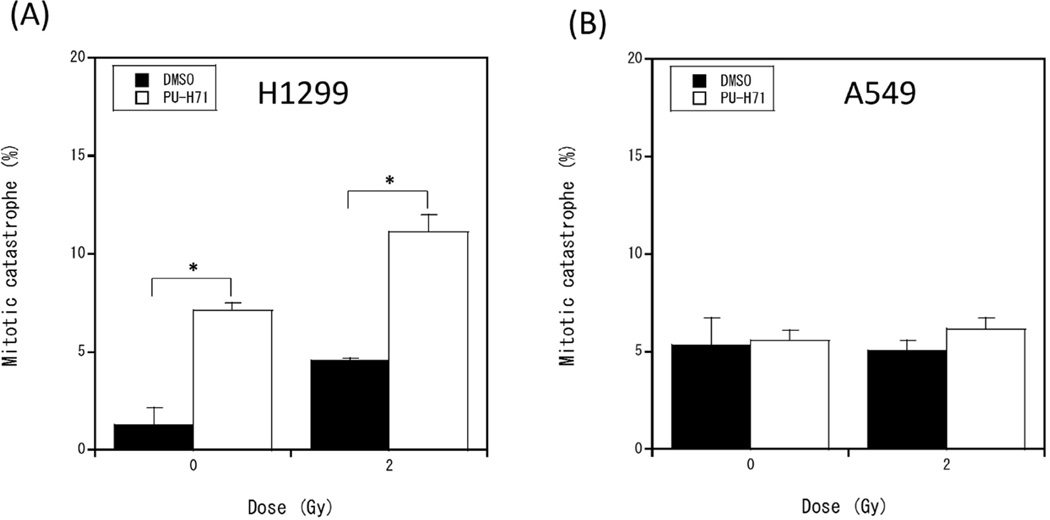
The results above demonstrate that PU-H71 inhibits DSB repair, which increases DSB persistence after carbon ion irradiation. DSBs induced by ionizing radiation can cause cell death or permanent cell cycle arrest through several genetically regulated pathways, including apoptosis, necrosis, and mitotic catastrophe [29,30]. To investigate whether PU-H71 treatment affects modes of cell death, we assessed changes in the percentages of sub-G1 cells (a marker of apoptotic cell death), and cells undergoing mitotic catastrophe. PU-H71 treatment alone increased the percentage of sub-G1 cells, and we observed a synergistic increase in sub-G1 cells with combined PU-H71 + carbon ion radiation in A549 cells (wild-type p53) 48 h after irradiation, but the combined treatment did not cause a further increase in sub-G1 cells in p53 mutant H1299 cells (Fig. 4 and Supplementary Fig. 6). Mitotic catastrophe is an important mode of cell death caused by radiation-induced DNA damage, especially in p53 null cells [22]. Mitotic catastrophe, assessed as altered nuclear morphology of DAPI stained cells (Supplementary Fig. 7), was increased in p53 null H1299 cells by PU-H71 treatment alone, and was further increased by combined carbon ion radiation (Fig. 5A). In contrast, neither PU-H71 nor carbon ion radiation treatments alone or in combination affected the percentage of cells suffering mitotic catastrophe in p53 wild-type A549 cells (Fig. 5B). These results demonstrate that mitotic catastrophe is one of the mechanisms of cell death by PU-H71 and carbon ions in p53–null H1299 cells and this may not be the case for A549 cells with wild type p53. Taken together, these results indicate that PU-H71 treatment of carbon ion irradiated cells can induce different modes of cell death in different cell types.
Fig. 4.
PU-H71 increases sub-G1 cell populations in irradiated human cancer cells. H1299 cells (A) and A549 cells (B) were pretreated PU-H71 for 24 h and irradiated with 3 Gy carbon ions. After 24 and 48 h, cells were fixed and stained with propidium iodide. The percentage of sub-G1 cells was determined by flow cytometry. Data represent mean ± SEM of two or three independent experiments. * indicates p < 0.05.
Fig. 5.
PU-H71 increases mitotic catastrophe in irradiated H1299 cells. H1299 cells (A) and A549 cells (B) were pretreated PU-H71 for 24 h and irradiated with 2 Gy carbon ions. After 72 h, cells were fixed and stained with DAPI. Mitotic catastrophe was analyzed by fluorescent microscopy, and nuclei with more than 2 distinct lobes were counted as cells undergoing mitotic catastrophe. Data represent mean ± SEM of two independent experiments. * indicates p < 0.05.
Discussion
The present study is the first demonstration that PU-H71 enhances radiosensitivity of human cancer cells to heavy ion radiation. These results complement a recent study indicating that PU-H71 sensitizes cancer cells to X-irradiation [16]. Our study also revealed that PU-H71 did not increase radiosensitivity of normal cells to heavy ion radiation, in contrast to cancer cells. In addition, we demonstrated that the increased sensitivity to heavy ion radiation imparted by PU-H71 can be accounted for by downregulation of both NHEJ and HR DSB repair pathways.
There is significant interest in inhibiting HR for cancer therapeutic gain [31–33]. We evaluated the expression levels of key DSB repair proteins to clarify the molecular mechanisms by which PU-H71 inhibits DSB repair in cancer cells. Similar to findings by Segawa, Fujii [16], we observed a marked reduction in RAD51 protein levels with PU-H71 treatment. RAD51 is a crucial factor in HR repair, and RAD51 overexpression correlates with radioresistance and poor clinical outcomes of NSCLC patients [34,35]. Given the critical role that RAD51 plays in both HR repair of DSBs and stalled or collapsed replication forks [36], PU-H71 or other Hsp90 inhibitors could improve the efficacy of a wide range of cancer therapeutic strategies.
NHEJ is another major DSB repair pathway that cancer cells engage to survive DNA damage induced by a range of cancer therapeutic strategies. We demonstrated that PU-H71 inhibited phosphorylation of two residues of DNA-PKcs that are important for its function in NHEJ. These phosphorylation events allow the ligase complex to bind broken ends at a late stage of NHEJ, enhancing DSB efficiency [23–25]. Thus, the ability of PU-H71 to reduce phosphorylation of DNA-PKcs should inhibit NHEJ. Similar to HR, NHEJ is another target that may improve therapeutic gain in cancer therapy [37]. In particular, inhibition of NHEJ and/or HR is an effective strategy for increasing tumor cell killing by low- and high-LET radiotherapy. For example, Zafar, Seidler [38] and Takahashi, Kubo [21] found that impairing HR or NHEJ repair increased radiosensitivity to high-LET heavy ion irradiation, and cells in which both HR and NHEJ repair were impaired showed the highest radiosensitivity [21]. In our study, we demonstrated that PU-H71 inhibits both HR and NHEJ repair pathways in heavy ion irradiated cancer cells. These results support the idea that PU-H71 will be useful in sensitizing tumors to heavy ion radiotherapy.
Although the present study does not precisely define the mechanisms by which PU-H71 affects RAD51 and DNA-PKcs, one clue is that both EGFR and phospho-ERK1/2 protein levels were decreased in PU-H71 treated cancer cells. Recent studies have shown that EGFR and ERK1/2 affect NHEJ and HR repair pathways [26–28]. Thus, blocking EGFR inhibits DNA-PKcs activation, especially phosphorylation of residue T2609, which reduces DSB repair efficiency in X-irradiated cells [39]. Inhibition of EGFR also reduces RAD51 levels and HR repair efficiency [40]. Moreover, inhibition of ERK1/2, which acts downstream of EGFR, reduces HR and NHEJ repair [40,41]. Thus, changes in EGFR and phospho-ERK1/2 protein by PU-H71 are likely contributors to PU-H71-induced NHEJ/HR repair inhibition in cancer cells, thereby enhancing sensitivity to heavy ion radiation.
Recent studies have highlighted the importance of cell death pathways for long-term tumor control. In particular, caspase 3-mediated apoptosis can trigger growth factor release, stimulating rapid growth of surviving tumor cells, a phenomenon long known as accelerated tumor repopulation [42,43]. We assessed apoptosis and mitotic catastrophe to determine whether PU-H71 alters the mode of cell death. We found that apoptosis was enhanced by PU-H71 in carbon ion irradiated A549 cells, but there was no effect in H1299 cells. Conversely, mitotic catastrophe was enhanced by PU-H71 in carbon ion irradiated H1299 cells, but not A549 cells. Prior reports demonstrated that mitotic catastrophe is enhanced in p53 deficient cells [44], and Amornwichet, Oike [22] suggested that mitotic catastrophe might play an important role in carbon ion induced cell death in p53 deficient cells. Our results are consistent with these studies, as p53 deficient H1299 cells showed increased mitotic catastrophe after treatment with carbon ion radiation or PU-H71, and it was further enhanced by the combined treatment, whereas mitotic catastrophe was unaffected by these treatments in p53 wild type A549 cells. Together, these results suggest that PU-H71 and carbon ion irradiation treatments induce different cell death pathway depending on cell types that may depend on factors such as p53 or K-Ras status which differ between H1299 and A549 cells. As these and other genetic factors may regulate cellular responses to PU-H71 and carbon ion radiation, further studies are needed to clarify the roles of p53, K-Ras, and other factors in these responses.
Prior studies have indicated that Hsp90 inhibitors such as PU-H71 and 17AAG display selective cytotoxic effects on tumor cells, with little or no effect on normal cells [11,45]. Our study reinforces this idea, as PU-H71 sensitized several cancer cells to carbon ion radiation, but it had no significant radiosensitizing effect on normal human fibroblasts. This cancer-selective effect of PU-H71 can be explained by differential binding affinity of PU-H71 to Hsp90 in cancer vs. normal cells. He, Zatorska [11] demonstrated that PU-H71 binds Hsp90 with far less affinity in normal cells than in cancer cells, resulting in selective cytotoxic effects. In conclusion, the cancer-selective effect of PU-H71, its effectiveness at relatively low concentrations, and its ability to inhibit both major DSB repair pathways, make this drug an attractive candidate to enhance the efficacy of heavy ion radiotherapy.
Supplementary Material
Acknowledgments
We thank Dr. Yoshitaka Matsumoto for valuable support during this work. We are grateful to the HIMAC operators for their technical support during carbon and iron irradiation. This work was supported by JSPS KAKENHI grants to R.O. (nos. 24249067 and 23390301), NIH R01 GM084020 to J.A.N., and was also a part of Research Project with Heavy Ions at NIRS-HIMAC.
Footnotes
Conflicts of interest statement
None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2016.08.029.
References
- 1.Kabakov AE, Kudryavtsev VA, Gabai VL. Hsp90 inhibitors as promising agents for radiotherapy. J Mol Med (Berl) 2010;88:241–247. doi: 10.1007/s00109-009-0562-0. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Bakanauskas VJ, Cerniglia GJ, Cheng Y, Bernhard EJ, Muschel RJ, et al. The Ras radiation resistance pathway. Cancer Res. 2001;61:4278–4282. [PubMed] [Google Scholar]
- 3.Pirollo KF, Hao Z, Rait A, Ho CW, Chang EH. Evidence supporting a signal transduction pathway leading to the radiation-resistant phenotype in human tumor cells. Biochem Biophys Res Commun. 1997;230:196–201. doi: 10.1006/bbrc.1996.5922. [DOI] [PubMed] [Google Scholar]
- 4.Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003;63:8984–8995. [PubMed] [Google Scholar]
- 5.Hirakawa H, Fujisawa H, Masaoka A, Noguchi M, Hirayama R, Takahashi M, et al. The combination of Hsp90 inhibitor 17AAG and heavy-ion irradiation provides effective tumor control in human lung cancer cells. Cancer Med. 2015;4:426–436. doi: 10.1002/cam4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machida H, Matsumoto Y, Shirai M, Kubota N. Geldanamycin, an inhibitor of Hsp90, sensitizes human tumour cells to radiation. Int J Radiat Biol. 2003;79:973–980. doi: 10.1080/09553000310001626135. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi M, Yu D, Hirayama R, Ninomiya Y, Sekine E, Kubota N, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem Biophys Res Commun. 2006;351:658–663. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 8.Chiosis G, Kang Y, Sun W. Discovery and development of purine-scaffold Hsp90 inhibitors. Expert Opin Drug Discov. 2008;3:99–114. doi: 10.1517/17460441.3.1.99. [DOI] [PubMed] [Google Scholar]
- 9.Grem JL, Morrison G, Guo XD, Agnew E, Takimoto CH, Thomas R, et al. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005;23:1885–1893. doi: 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 10.Egorin MJ, Rosen DM, Wolff JH, Callery PS, Musser SM, Eiseman JL. Metabolism of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) by murine and human hepatic preparations. Cancer Res. 1998;58:2385–2396. [PubMed] [Google Scholar]
- 11.He H, Zatorska D, Kim J, Aguirre J, Llauger L, She Y, et al. Identification of potent water soluble purine-scaffold inhibitors of the heat shock protein 90. J Med Chem. 2006;49:381–390. doi: 10.1021/jm0508078. [DOI] [PubMed] [Google Scholar]
- 12.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A. 2009;106:8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallerne C, Prola A, Lemaire C. Hsp90 inhibition by PU-H71 induces apoptosis through endoplasmic reticulum stress and mitochondrial pathway in cancer cells and overcomes the resistance conferred by Bcl-2. Biochim Biophys Acta. 2013;1833:1356–1366. doi: 10.1016/j.bbamcr.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Allen C, Borak TB, Tsujii H, Nickoloff JA. Heavy charged particle radiobiology: using enhanced biological effectiveness and improved beam focusing to advance cancer therapy. Mutat Res. 2011;711:150–157. doi: 10.1016/j.mrfmmm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:953–964. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 16.Segawa T, Fujii Y, Tanaka A, Bando S, Okayasu R, Ohnishi K, et al. Radiosensitization of human lung cancer cells by the novel purine-scaffold Hsp90 inhibitor, PU-H71. Int J Mol Med. 2014;33:559–564. doi: 10.3892/ijmm.2013.1594. [DOI] [PubMed] [Google Scholar]
- 17.Okayasu R, Okada M, Okabe A, Noguchi M, Takakura K, Takahashi S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat Res. 2006;165:59–67. doi: 10.1667/rr3489.1. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Wang X, Zhang P, Wang Y. The Ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair (Amst) 2008;7:725–733. doi: 10.1016/j.dnarep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Yajima H, Fujisawa H, Nakajima NI, Hirakawa H, Jeggo PA, Okayasu R, et al. The complexity of DNA double strand breaks is a critical factor enhancing end-resection. DNA Repair (Amst) 2013;12:936–946. doi: 10.1016/j.dnarep.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Gerelchuluun A, Manabe E, Ishikawa T, Sun L, Itoh K, Sakae T, et al. The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat Res. 2015;183:345–356. doi: 10.1667/RR13904.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi A, Kubo M, Ma H, Nakagawa A, Yoshida Y, Isono M, et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat Res. 2014;182:338–344. doi: 10.1667/RR13782.1. [DOI] [PubMed] [Google Scholar]
- 22.Amornwichet N, Oike T, Shibata A, Ogiwara H, Tsuchiya N, Yamauchi M, et al. Carbon-ion beam irradiation kills X-ray-resistant p53-null cancer cells by inducing mitotic catastrophe. PLoS ONE. 2014;9:e115121. doi: 10.1371/journal.pone.0115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 24.Jette N, Lees-Miller SP. The DNA-dependent protein kinase: A multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog Biophys Mol Biol. 2015;117:194–205. doi: 10.1016/j.pbiomolbio.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis AJ, Chen BP, Chen DJ. DNA-PK: a dynamic enzyme in a versatile DSB repair pathway. DNA Repair (Amst) 2014;17:21–29. doi: 10.1016/j.dnarep.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13:6555–6560. doi: 10.1158/1078-0432.CCR-07-1610. [DOI] [PubMed] [Google Scholar]
- 27.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Wang H, Yang ES, Arteaga CL, Xia F. Erlotinib attenuates homologous recombinational repair of chromosomal breaks in human breast cancer cells. Cancer Res. 2008;68:9141–9146. doi: 10.1158/0008-5472.CAN-08-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 31.Chernikova SB, Game JC, Brown JM. Inhibiting homologous recombination for cancer therapy. Cancer Biol Ther. 2012;13:61–68. doi: 10.4161/cbt.13.2.18872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helleday T. Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis. 2010;31:955–960. doi: 10.1093/carcin/bgq064. [DOI] [PubMed] [Google Scholar]
- 33.Lok BH, Powell SN. Molecular pathways: understanding the role of Rad52 in homologous recombination for therapeutic advancement. Clin Cancer Res. 2012;18:6400–6406. doi: 10.1158/1078-0432.CCR-11-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vispe S, Cazaux C, Lesca C, Defais M. Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res. 1998;26:2859–2864. doi: 10.1093/nar/26.12.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao GB, Wu YL, Yang XN, Zhong WZ, Xie D, Guan XY, et al. High-level expression of Rad51 is an independent prognostic marker of survival in non-small-cell lung cancer patients. Br J Cancer. 2005;93:137–143. doi: 10.1038/sj.bjc.6602665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen C, Ashley AK, Hromas R, Nickoloff JA. More forks on the road to replication stress recovery. J Mol Cell Biol. 2011;3:4–12. doi: 10.1093/jmcb/mjq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastwa E, Malinowski M. Non-homologous DNA end joining in anticancer therapy. Curr Cancer Drug Targets. 2007;7:243–250. doi: 10.2174/156800907780618284. [DOI] [PubMed] [Google Scholar]
- 38.Zafar F, Seidler SB, Kronenberg A, Schild D, Wiese C. Homologous recombination contributes to the repair of DNA double-strand breaks induced by high-energy iron ions. Radiat Res. 2010;173:27–39. doi: 10.1667/RR1910.1. [DOI] [PubMed] [Google Scholar]
- 39.Toulany M, Kasten-Pisula U, Brammer I, Wang S, Chen J, Dittmann K, et al. Blockage of epidermal growth factor receptor-phosphatidylinositol 3-kinase-AKT signaling increases radiosensitivity of K-RAS mutated human tumor cells in vitro by affecting DNA repair. Clin Cancer Res. 2006;12:4119–4126. doi: 10.1158/1078-0432.CCR-05-2454. [DOI] [PubMed] [Google Scholar]
- 40.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730–738. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046–1053. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 42.Allen CP, Tinganelli W, Sharma N, Nie J, Sicard C, Natale F, et al. DNA Damage Response Proteins and Oxygen Modulate Prostaglandin E2 Growth Factor Release in Response to Low and High LET Ionizing Radiation. Front Oncol. 2015;5:260. doi: 10.3389/fonc.2015.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ianzini F, Mackey MA. Mitotic catastrophe. In: Gewirtz DA, Holt SE, Grant S, editors. Apoptosis, senescence, and cancer. Humana Press; 2007. pp. 73–91. [Google Scholar]
- 45.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.