Abstract
Breast cancer is the most common cancer in women and the second leading cause of cancer deaths in women. The key to surviving breast cancer is early detection and treatment. Current technologies rely heavily on imaging of the breast, and although considered the gold standard, they have their limitations. There is a need for a more accurate screening test for women of all ages, which can detect the cancer at a cellular level and before metastasis. There have been extensive studies into markers for breast cancer including protein and nucleic acid biomarkers, but to date, these have been unsuccessful. A growing field of interest is the association between breast cancer (tissue and cells) and lipids, which is documented in the literature, and may be considered as a leading candidate in the breast cancer detection space.
Keywords: breast cancer, lipids, phospholipids, blood, plasma, biomarkers
Breast Cancer
The extent of breast cancer
The most common type of cancer among women is breast cancer, with over 1.7 million women worldwide diagnosed with the disease annually,1 and of these women, 35% will lose their lives to the disease.2 Although breast cancer is mostly a disease of females, 1 in 1100 males may also develop the disease.3
Incidence by age
Breast cancer occurs in women of all ages, although, as a woman ages, her risk of developing breast cancer increases. Women between the ages of 20 and 29 years account for only 0.3% of breast cancer cases. Around 20% of breast tumors are found in women younger than 50 years, and 40% of breast tumors are found in women aged 65 years and older.4 About 80% of women with breast cancer are older than 50 years at the time of diagnosis.
Current recommended screening
Ademuyiwa et al5 have recently stated that screening for breast cancer, via annual mammography and clinical breast examination, should begin at the age 40 years for average-risk women. Breast self examination is an additional option, although it is not recommended in some countries.6 For women under the age of 40 years with no other risk factors, routine mammography is not recommended. Instead, these women are encouraged to undergo clinical breast examination every three years, with breast self examination being optional. Given this, Ademuyiwa et al state that most malignancies in women under 40 years will be detected by patients themselves. The limitation of these methods is that they rely on a visible or palpable lump for detection, which means that in practice the tumor has to be of a minimum size (at least 5–10 mm), by which time it may have metastasized.
Mammography is not recommended for younger women as their breasts are denser and the identification of masses is less obvious in breast imaging.7,8 Since most breast cancers occur in older women, this is not considered a major problem, but for young women who have a genetic risk factor for breast cancer, the relative lack of effectiveness of mammograms is a serious issue because breast cancer often develops at a younger age in these women9 and is often more aggressive than in older women.10–12
The importance of early detection
Although the overall incidence of breast cancer has been increasing for more than two decades, there has been almost a 30% reduction in mortality beginning in 1990.13 The two factors that have been attributed to improved mortality rates are14 early detection (primarily by mammography screening) and more effective and increased application of antihormonal drugs and chemotherapy. This has been the basis for developing improved methods for the early detection of various forms of breast cancer, prior to it metastasizing, in order to maximize the treatment outcomes.15 It is estimated that 40% of breast cancer patients have regional (stages II–III) or distant (stage IV) spread of their disease at the time of diagnosis16 and this has changed little in the past 20 years despite the introduction of screening mammography in many countries for women in the target age group of 50–69 years. If breast cancer could be reliably detected prior to metastasising, there would be a significant reduction in individual mortality in the community.17 This is not achievable by current breast screening technologies.
A better screening test is necessary
Simply stated, “if a tool, such as mammography, with only 40–70% sensitivity can reduce breast cancer mortality, then not only is early detection valid, but it is also a more powerful approach than ever imagined”.18 The impact of a technology that has 90% sensitivity or more and has the potential to find 30%–60% of currently undetectable cancers19 would have the potential to achieve an enormous reduction in breast cancer mortality.
A screening test should satisfy several criteria in order to reach the guidelines of public health and to reduce the morbidity and mortality of the diseased or symptomatic population. The criteria are as follows20:
high precision and accuracy,
high sensitivity and specificity,
useful for medical decision making,
high predictive value,
favorable cost/benefit ratio, and
high throughput.
In addition, a screening test should be able to detect the disease at an early enough stage to allow for effective treatment.21,22 Attaining all of these requirements, while achievable in theory, is still highly challenging considering today’s current breast cancer screening technologies.
A biological marker, the measurement of which is objective, provides the direction required to develop a screening test for breast cancer that can address these requirements.
The Association of Altered Composition of Phospholipids and Breast Cancer
Lipids
Lipids, also known as fatty acids, are carboxylic acids composed of a carboxyl group and hydrocarbon chain with a polar hydrophilic end and a nonpolar hydrophobic end.23 Lipids are known to play a multitude of roles in cellular biology. They make up at least 50% of the cellular membrane, serving as structural molecules,24 and are influential in signaling pathways within cells through their interaction with transmembrane proteins.25–27
There is considerable evidence that some lipids are elevated in breast cancer cells and tissue.28–38 A study on breast and tumor tissues has found that the distribution of individual lipid species varied and was “not uniform, reflecting microenvironment differences” due to the integration of tumor tissue into healthy breast tissue.39 The same group also found that the level of a particular lipid was 28% higher in high-grade tumor tissue than in low-grade tumor tissue.40 Mimmi et al41 also reported highly increased levels of phosphocho-line (PCho), total choline, and PCho/glycerophosphocholine ratio in tissue samples taken from patients with breast cancer than in healthy tissue. It has also been reported that there is a significant difference in serum lipid content between breast cancer patients and disease-free individuals, in particular, in a class of lipids called phospholipids.42–44
Phospholipids as biomarkers of breast cancer
Phospholipids are a class of lipids consisting of two fatty acyl molecules esterified at the sn-1 and sn-2 positions of glycerol and contain a head group linked by a phosphate residue at the sn-3 position.45 They are composed of hydrophobic fatty acyl chains and a hydrophilic head group, which defines the phospholipid species (Fig. 1).
Figure 1.
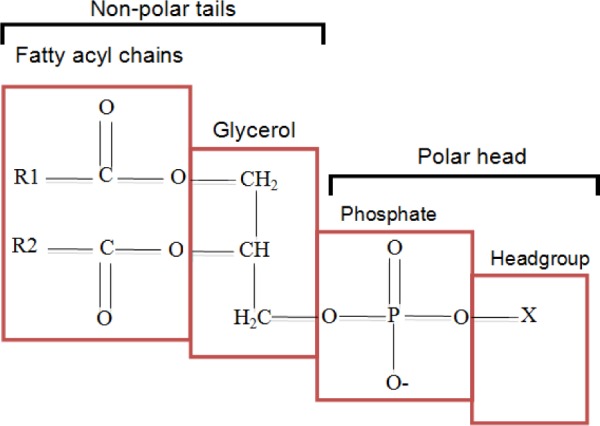
Schematic representation of a phospholipid species structure. Adapted from Ref. 45.
Phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin are the most dominant phospholipids in the majority of biological membranes, comprising up to 80% of the membrane.46 Most studies report an increase in phospholipid metabolism in breast cancer tissue, particularly PC and PE or their precursor molecules (PCho and phosphoethanolamine).35,47–49 Some studies have found specific changes in choline metabolism that were linked to more aggressive carcinomas.50,51 It has been proposed that the altered cellular lipid structure is associated with altered cellular functions, such as protein trafficking, which promotes the onset of cancer or contributes to the progression of the disease.52–54
Measuring phospholipids using mass spectrometry
Lipid extraction from biological samples requires the use of solvent. Membrane-bound lipids need polar solvents (ethanol and methanol) to disrupt the hydrogen bonds and to release the lipids, whereas other lipids can be extracted using nonpolar solvents such as chloroform and diethyl ether.55
Once extracted, species identification and analysis are currently performed using mass spectrometry, as this technique allows the most sensitive and selective identification and quantification of a variety of lipids.56
Mass spectrometry measures the molecular mass of a sample using the following three functional components: an ionization source, an analyzer, and a detector.57 The sample is injected into the ionization source where molecules of the sample are ionized making them easier to manipulate than neutral molecules.58 The ions then enter into the highly vacuumed region of the spectrometer, the analyzer, and the detector. This vacuum ensures that the ions travel through the instrument without an interference from air molecules. There are two analyzers; the first separates the ions according to their mass-to-charge ratio (m/z), and the second monitors specific fragment ions. They are then identified on the detector as a signal on an attached computer. The m/z ratios along with the relative abundance present the sample as an m/z spectrum and allow qualitative and quantitative detections. Raw data from the mass spectrometer are processed using the commercial software, which contains information about the masses identified from the precursor scans and subsequent analysis, and the spectra are matched against a lipid library.
Lipogenesis and breast cancer
The loss of breast cancer susceptibility gene 1 (BRCA1) is associated with breast cancer.59 BRCA1 is a tumor suppressor gene and is also an inhibitor of lipogenesis, and the loss of BRCA1 has been shown to increase lipid production in breast cancer cells.60 In addition, it has been demonstrated that the activation of fatty acid synthase expression and concomitant lipid synthesis is a common event in breast cancer.61
There is further evidence that increased lipogenesis is closely linked to tumorigenesis in breast cancer. Chajes et al29 found that mechanisms specifically related to malignant transformation and tumor progression influence the membrane lipid profile of breast carcinoma as determined by thin layer chromatography.
In vitro association of lipids and breast cancer cells
Increased expression levels of specific choline transporters and of PCho occur in breast cancer cells than in normal mammary epithelial cells.28,30,32,37,38,62
Singer et al36 found a 16–19-fold increase in PCho content in two primary breast cancer cell lines and a 27-fold increase in PCho content in a metastatic breast cancer cell line compared with normal breast epithelial cells. There are other indications that an altered phospholipid profile correlates with alteration in tumor characteristics. Total phospholipids in malignant breast cancer cell lines differ between hormone-sensitive and highly hormone-resistant tumors.63 In particular, two phospholipid components, a PC and a PE, that were absent or at very low levels in hormone-sensitive cells were significantly increased in highly hormone-resistant cell lines.
Phospholipids in body fluids associated with breast cancer
Levels of phospholipids have been reported in a small five-patient pilot study in urine64,65 and serum or plasma43,66–68 of patients with breast cancer. The urine analysis showed that PC, PE, and two phosphatidylserine molecules (18:1/18:1 and 18:2/18:0) were significantly increased in some of the breast cancer patients and decreased to baseline levels following surgery. Feldman and Carter’s66 study failed to show any difference in the levels of phospholipids between women with breast cancer and healthy women, but in later studies, significant differences were found. In Hammad et al’s43 study, the most significant differences in lipid profiles among disease-free and cancer subjects were attributed to three PCho species (precursors to PC) and to three unidentified fatty acid species. Yang et al67 also reported that specific phospholipids found in the plasma of patients with benign breast tumors, malignant breast tumors, and healthy controls were able to discriminate between the groups and suggested that these phospholipids have potential in the clinical diagnostic space. The concentration of serum lipids has been shown to be affected following treatment of the disease as Ray et al68 found that those lipids that were elevated in breast cancer decreased after treatment.
Plasma is known to contain multiple lipoprotein pools, each consisting of many lipid classes that contain up to thousands of separate lipid species.69 Their composition is highly influenced by dietary sources. Meikle et al showed that many of the phospholipid classes of interest in the association of changes of levels of phospholipids with breast cancer are altered following consumption of soy or dairy diets. For example, they reported that plasma PC, PE, phosphatidylinositol, and phosphatidylglycerol increased significantly after a dairy-based meal. However, after a soy-based meal, several phospholipids decreased, in particular sphingomyelin and the ether-linked and lysophospholipids.70 This needs to be taken into account when attempting to determine an association between plasma phospholipids and diseases such as breast cancer.
Therefore, to be able to discern a consistent pattern of phospholipid species across different breast cancer types above the noise of dietary lipids is a significant challenge. Exosomes from breast tumors provide a more specific source of cancer-associated lipids than whole serum.
Exosomes
Extracellular vesicles (EVs) are produced by the outward budding and release of lipid-bound particles from cells into the extracellular environment.71,72 One type of these EVs is of endosomal origin, called exosomes. Exosomes are released into the extracellular environment when an endocytic invagination forms a multivesicular body, which fuses with the plasma membrane.71–74
Another type of EVs is derived from the plasma membrane, called microvesicles, which are directly pinched off the plasma membrane toward the extracellular environment.71–74 These extracellular vesicles have been postulated to have a role in intercellular communication as transport vehicles of proteins, lipids, and RNA75 and also appear to be involved in tumor progression.71 They have been suggested to facilitate malignancy, invasiveness, and the evasion of the immune response.76–79
The cellular origin of these extracellular vesicles determines their make up, and once they are released into the extracellular environment, they may be involved in the transfer of molecules between cells and also enable the deposit or removal of molecules at distal sites.71 As a result, they have been identified in numerous bodily fluids including blood, urine, breast milk, and saliva, which make them a candidate as markers for identifying intracellular changes.80–82 Once these fluids have been collected, exosomes can be easily isolated using differential ultracentrifugation.80 The ubiquitous nature of exosomes in bodily fluids makes them ideal for use in diagnostic biomarker studies.80
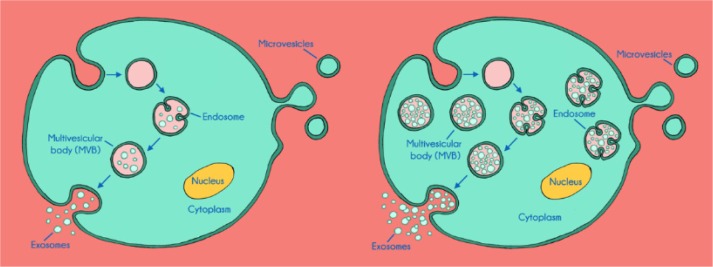
It has been observed that there are significantly elevated exosome concentrations in the blood of patients with cancer than in that of controls. This could be due either to an increased production of exosomes per vesicle (endosome/multivesicular body)83,84 in cancer cells or to an increased production of these endosomes and multivesicular bodies in cancer cells (see Fig. 2).
Figure 2.
Schematic illustration of exosome production in a normal cell (left) versus exosome production in a cancer cell (right), which illustrates the elevation of exosome production and the potential amplification of metabolites.
Tumor-derived exosomes have a distinct protein and lipid composition resembling that of the cells from which they are derived.85 Phospholipids isolated from exosomes in plasma from patients with breast cancer are reported to be different to those identified in plasma from patients with lung cancer, which are distinguishable from healthy individuals.86 Therefore, this makes phospholipids from exosomes a potentially specific biomarker for the detection of breast cancer, possibly even at an early stage.
Therefore, the lipid composition of blood-borne exosomes originating from tumor cells is likely to reflect tumor-specific membrane alterations independent of dietary lipids in the blood. Smith et al87 recently reported that cancerous cell-derived exosomes are relatively depleted in cholesterol and enriched in phospholipid compared to noncancerous cell-derived exosomes.
In support of the influence of dietary lipids as a confounding variable in determining an association between phospholipids and the presence of breast cancer, measurement of the phospholipids in the plasma from fasting breast cancer patients was able to accurately distinguish cancer patients from healthy controls.44 This indicates that when dietary lipids are removed as a confounder, the endogenous phospholipids provide specificity for the detection of the presence of breast cancer. It is likely that these endogenous phospholipids originate from exosomes shed from the tumors.
Conclusion
Despite the widespread use of mammography, there is an acknowledged need for a more reliable screening test for breast cancer. The optimal biomarker would be one that could identify specific molecular changes in the body shortly after a breast tumor is formed. Most biomarker studies have failed to deliver an accurate assay, probably due to the heterogeneity of the disease, and the low accuracy of assays that use one or a small number of biomarkers.88 Panels consisting of multiple biomarkers are likely to be a more sensitive and specific approach to detect the disease.89 Circulating endogenous phospholipids in the plasma of breast cancer patients originating from tumor-derived exosomes may represent a novel class of biomarkers that could be used as the basis of a blood-based screening test for the detection of the disease. Preliminary reports indicate that a panel of phospholipids is required to achieve high levels of accuracy. More work is needed to overcome issues of scalability of exosome isolation and mass spectroscopy multivariate analysis before such a screening test could be practicable. A combination of mammography and phospholipid analysis may result in an increased accuracy and earlier detection of disease, with a resulting significant improvement in morbidity and mortality.
Acknowledgments
The authors wish to thank BCAL Diagnostics Pty Ltd for their support in funding this review.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1037 words, excluding any confidential comments to the academic editor.
FUNDING: This review was funded by BCAL Diagnostics Pty Ltd (formerly SBC Research Pty Ltd).
COMPETING INTERESTS: Dr. Dharmica Mistry is employed by BCAL Diagnostics Pty Ltd. Dr. Dharmica Mistry and Dr. Peter French are shareholders in BCAL Diagnostics Pty Ltd. BCAL Diagnostics is developing a commercial blood test for breast cancer based on detection of circulating phospholipids.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Analyzed the data: DM, PF. Wrote the first draft of the manuscript: PF. Contributed to the writing of the manuscript: DM. Agree with manuscript results and conclusions: DM, PF. Jointly developed the structure and arguments for the paper: DM, PF. Made critical revisions and approved final version: DM, PF. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon: International Agency for Research on Cancer; 2014. Available at: http://globocan.iarc.fr. [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Society AC . Cancer Facts and Figures 2016. Atlanta: American Cancer Society, Inc; 2016. Available at: http://www.cancer.org/cancer/breastcancerinmen/detailedguide/breast-cancer-in-men-key-statistics. [Google Scholar]
- 4.Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 5.Ademuyiwa FO, Cyr A, Ivanovich J, Thomas MA. Managing breast cancer in younger women: challenges and solutions. Breast Cancer (Dove Med Press) 2016;8:1–12. doi: 10.2147/BCTT.S68848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackshaw A. Screening for breast cancer in young women using breast self-examination. In: PRACTITIONERS RCOGHannaford PC, Webb AMC, editors. Evidence-Guided Prescribing of the Pill. Lancs: Parthenon Publishing Group; 1996. [Google Scholar]
- 7.Elwood J, Cox B, Richardson AK. The effectiveness of breast cancer screening by mammography in younger women. Online J Clin Trials. 1993;2:Doc 34. [PubMed] [Google Scholar]
- 8.Yankaskas B, Haneuse S, Kapp JM, et al. Performance of first mammography examination in women younger than 40 years. J Natl Cancer Inst. 2010;102:692. doi: 10.1093/jnci/djq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastiaannet E, Liefers GJ, De Craen AJ, et al. Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat. 2010;124:801. doi: 10.1007/s10549-010-0898-8. [DOI] [PubMed] [Google Scholar]
- 10.Anders C, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foxcroft L, Evans EB, Porter AJ. The diagnosis of breast cancer in women younger than 40. Breast. 2004;13(4):297–306. doi: 10.1016/j.breast.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4:7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopans DB. Why the critics of screening mammography are wrong. Diagn Imaging. 2009;31:18–24. [Google Scholar]
- 14.Berry D, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 15.Ruffin M, Gorenflo DW, Woodman B. Predictors of screening for breast, cervical, colorectal, and prostatic cancer among community-based primary care practices. J Am Board Fam Pract. 2000;13:1–10. doi: 10.3122/jabfm.13.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin Oncol. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 17.Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer. 2003;3:243–252. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 18.Hollingsworth A. The rationale for a blood test to detect early breast cancer. In: CENTER MWSCAMCR, editor. Mercy Health System of Oklahoma. Oklahoma: Mercy Health System of Oklahoma; 2005. [Google Scholar]
- 19.Kuhl C, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–492. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 20.Galen R. Application of the predictive value model in the analysis of test effectiveness. Clin Lab Med. 1982;2:685–699. [PubMed] [Google Scholar]
- 21.Knottneurus J. The evidence base of clinical diagnosis: evaluation of diagnostic procedures. BMJ. 2002;324:477–480. doi: 10.1136/bmj.324.7335.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sackett D, Haynes R. The architecture of diagnostic research. BMJ. 2002;324(7336):539–541. doi: 10.1136/bmj.324.7336.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahy E, Subramaniam S, Brown H, et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Yeung T, Grinstein S. Lipid signaling and the modulation of surface charge during phagocytosis. Immunol Rev. 2007;219:17–36. doi: 10.1111/j.1600-065X.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 25.Hannun Y, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 26.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wymann M, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 28.Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59:80–84. [PubMed] [Google Scholar]
- 29.Chajes V, Lanson M, Fetissof F, Lhuillery C, Bougnoux P. Membrane fatty acids of breast carcinoma: contribution of host fatty acids and tumor properties. Int J Cancer. 1995;63:169–175. doi: 10.1002/ijc.2910630204. [DOI] [PubMed] [Google Scholar]
- 30.Eliyahu G, Kreizman T, Degani H. Phosphocholine as a biomarker of breast cancer: molecular and biochemical studies. Int J Cancer. 2007;120:1721–1730. doi: 10.1002/ijc.22293. [DOI] [PubMed] [Google Scholar]
- 31.Hilvo M, Denkert C, Lehtinen L, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71:3236–3245. doi: 10.1158/0008-5472.CAN-10-3894. [DOI] [PubMed] [Google Scholar]
- 32.Katz-Brull R, Degani H. Kinetics of choline transport and phosphorylation in human breast cancer cells, NMR application of the zero trans method. Anticancer Res. 1996;16:1375–1380. [PubMed] [Google Scholar]
- 33.Katz-Brull R, Seger D, Rivenson-SEGAL D, Rushkin E, Degani H. Metabolic markers of breast cancer: enhanced choline metabolism and reduced choline-ether-phospholipid synthesis. Cancer Res. 2002;62:1966–1970. [PubMed] [Google Scholar]
- 34.Monteggia E, Colombo I, Guerra A, Berra B. Phospholipid distribution in murine mammary adenocarcinomas induced by activated neu oncogene. Cancer Detect Prev. 2000;24:207–211. [PubMed] [Google Scholar]
- 35.Podo F. Tumour phospholipid metabolism. Review article. NMR Biomed. 1999;12:413–439. doi: 10.1002/(sici)1099-1492(199911)12:7<413::aid-nbm587>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Singer S, Souza K, Thilly WG. Pyruvate utilization, phosphocholine and adenosine triphosphate (ATP) are markers of human breast tumor progression: a 31P and 13C-nuclear magnetic resonance (NMR) spectroscopy study. Cancer Res. 1995;55:5140–5145. [PubMed] [Google Scholar]
- 37.Ting Y, Sherr D, Degani H. Variations in the energy and phospholipid metabolism in normal and cancer human mammary epithelial cells. Anticancer Res. 1996;16:1381–1388. [PubMed] [Google Scholar]
- 38.Tse G, Yeung DK, King AD, Cheung HS, Yang WT. In vivo proton magnetic resonance spectroscopy of breast lesions: an update. Breast Cancer Res Treat. 2007;104:249–255. doi: 10.1007/s10549-006-9412-8. [DOI] [PubMed] [Google Scholar]
- 39.Smith TAD, Glaholm J, Leach MO, Machin L, Mccready VR. The effect of intra-tumour heterogeneity on the distribution of phosphorus-containing metabolites within human breast tumours: an in vitro study using 31P NMR spetroscopy. NMR Biomed. 1991;4:262–267. doi: 10.1002/nbm.1940040603. [DOI] [PubMed] [Google Scholar]
- 40.Smith TAD, Bush C, Jameson C, et al. Phospholipid metabolites, prognosis and proliferation in human breast carcinoma. NMR Biomed. 1993;6:318–323. doi: 10.1002/nbm.1940060506. [DOI] [PubMed] [Google Scholar]
- 41.Mimmi MC, Finato N, Pizzolato G, et al. Absolute quantification of choline-related biomarkers in breast cancer biopsies by liquid chromatography electro-spray ionization mass spectrometry. Anal Cell Pathol (Amst) 2013;36:71–83. doi: 10.3233/ACP-130082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexopoulos CG, Blatsios B, Avgerinos A. Serum lipids and lipoprotein disorders in cancer patients. Cancer. 1987;60:3065–3070. doi: 10.1002/1097-0142(19871215)60:12<3065::aid-cncr2820601234>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Hammad LA, Wu G, Saleh MM, et al. Elevated levels of hydroxylated phosphocholine lipids in the blood serum of breast cancer patients. Rapid Commun Mass Spectrom. 2009;23:863–876. doi: 10.1002/rcm.3947. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Chen H, Dai M, et al. Plasma lipidomics profiling identified lipid biomarkers in distinguishing early-stage breast cancer from benign lesions. Oncotarget. 2016;7(24):36622–36631. doi: 10.18632/oncotarget.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly KAJR. Phospholipid Biosynthesis. 2011. Available at AOCS Lipid Library: http://lipidlibrary.aocs.org/Biochemistry/content.cfm?ItemNumber=39191.
- 46.Fajardo V, Mc Meekin L, Le Blanc PJ. Influence of phospholipid species on membrane fluidity: a meta-analysis for a novel phospholipid fluidity index. J Membr Biol. 2011;244:97–103. doi: 10.1007/s00232-011-9401-7. [DOI] [PubMed] [Google Scholar]
- 47.Cairns R, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 48.de Certaines J, Larsen VA, Podo F, Carpinelli G, Briot O, Henriksen O. In vivo 31P MRS of experimental tumours. NMR Biomed. 1993;6:345–365. doi: 10.1002/nbm.1940060602. [DOI] [PubMed] [Google Scholar]
- 49.Menendez J, Lupu R, Colomer R. Targeting fatty acid synthase: potential for therapeutic intervention in Her-2/neu-overexpressing breast cancer. Drug News Perspect. 2005;18:375. doi: 10.1358/dnp.2005.18.6.927929. [DOI] [PubMed] [Google Scholar]
- 50.Glunde K, Ackerstaff E, Mori N, Jacobs MA, Bhujwalla M. Choline phospholipid metabolism in cancer: consequences for molecular pharmaceutical interventions. Mol Pharm. 2006;3:496–506. doi: 10.1021/mp060067e. [DOI] [PubMed] [Google Scholar]
- 51.Yonekubo Y, Wu P, Esechie A, Zhang Y, DU G. Characterization of new serum biomarkers in breast cancer using lipid microarrays. Tumor Biol. 2010;31:181–187. doi: 10.1007/s13277-010-0027-7. [DOI] [PubMed] [Google Scholar]
- 52.Ackerstaff E, Glunde K, Bhujwalla ZM. Choline phospholipid metabolism: a target in cancer cells? J Cell Biochem. 2003;90:525–533. doi: 10.1002/jcb.10659. [DOI] [PubMed] [Google Scholar]
- 53.Fernandis AZ, Wenk MR. Identification of an individual lipid, a class of lipids or a unique fingerprint of lipids species is a first step in development of new biomarkers for applications in cancer and other diseases. J Chromatogr B. 2009;877:2830–2835. [Google Scholar]
- 54.Patra S. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Han XA, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Köfeler HC, Fauland A, Rechberger GN, Trötzmüller M. Mass spectrometry based lipidomics: an overview of technological platforms. Metabolites. 2012;2:19–38. doi: 10.3390/metabo2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashcroft AE. An Introduction to Mass Spectrometry. The University of Leeds; 2014. Available at: http://www.astbury.leeds.ac.uk/facil/MStut/mstutorial.htm. [Google Scholar]
- 58.Scott RPW. Essential Information for the Analytical Chemist Analytical Spectroscopy. 2014. Available at: http://www.analyticalspectroscopy.net.
- 59.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 60.Moreau K, Dizin E, Ray H, et al. BRCA1 affects lipid synthesis through its interaction with acetyl-CoA carboxylase. J Biol Chem. 2006;281:3172–3181. doi: 10.1074/jbc.M504652200. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Morin PJ, Han WF, et al. Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element binding protein-1c. Exp Cell Res. 2003;282:132–137. doi: 10.1016/s0014-4827(02)00023-x. [DOI] [PubMed] [Google Scholar]
- 62.Katz-Brull R, Margalit R, Degani H. Differential routing of choline in implanted breast cancer and normal organs. Magn Reson Med. 2001;46:31–38. doi: 10.1002/mrm.1157. [DOI] [PubMed] [Google Scholar]
- 63.Sterin M, Cohen JS, Ringel I. Hormone sensitivity is reflected in the phospholipid profiles of breast cancer cell lines. Breast Cancer Res Treat. 2004;87:1–11. doi: 10.1023/B:BREA.0000041572.07837.ec. [DOI] [PubMed] [Google Scholar]
- 64.Kim H, Min HK, Kong G, Moon MH. Quantitative analysis of phosphatidylcholinesand phosphatidylethanolamines in urine of patients with breast cancer by nanoflow liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem. 2009;393:1649–1656. doi: 10.1007/s00216-009-2621-3. [DOI] [PubMed] [Google Scholar]
- 65.Min HK, Kong G, Moon MH. Quantitative analysis of urinary phospholipids found in patients with breast cancer by nanoflow liquid chromatography-tandem mass spectrometry: II. Negative ion mode analysis of four phospholipid classes. Anal Bioanal Chem. 2010;396:1273–1280. doi: 10.1007/s00216-009-3292-9. [DOI] [PubMed] [Google Scholar]
- 66.Feldman EB, Carter AC. Circulating lipids and lipoproteins in women with metastatic breast carcinoma. J Clin Endocrinol Metab. 1971;33:8–13. doi: 10.1210/jcem-33-1-8. [DOI] [PubMed] [Google Scholar]
- 67.Yang L, Cui XG, Zhang NN, et al. Comprehensive lipid profiling of plasma in patients with benign breast tumor and breast cancer reveals novel biomarkers. Anal Bioanal Chem. 2015;407:5065–5077. doi: 10.1007/s00216-015-8484-x. [DOI] [PubMed] [Google Scholar]
- 68.Ray A, Jain D, Yadav R, et al. Effect of cancer treatment modalities on serum lipids and lipoproteins among women with carcinoma of the breast. Indian J Physiol Pharmacol. 2001;45:337–344. [PubMed] [Google Scholar]
- 69.Ruiz-Cabello J, Cohen JS. Phospholipid metabolites as indicators of cancer cell function. NMR Biomed. 1992;5:226–233. doi: 10.1002/nbm.1940050506. [DOI] [PubMed] [Google Scholar]
- 70.Meikle PJ, Barlow CK, Mellett NA, et al. Postprandial plasma phospholipids in men are influenced by the source of dietary fat. J Nutr. 2015;145:2012–2018. doi: 10.3945/jn.115.210104. [DOI] [PubMed] [Google Scholar]
- 71.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’souza-schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:932–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wendler F, Bota-Rabassedas N, Franch-Marro X. Cancer becomes wasteful: emerging roles of exosomes† in cell-fate determination. J Extracell Vesicles. 2013;2:10. doi: 10.3402/jev.v2i0.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Dolo V, D’ascenzo S, Violini S, et al. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis. 1999;17:131–140. doi: 10.1023/a:1006500406240. [DOI] [PubMed] [Google Scholar]
- 78.Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004;64:7045–7049. doi: 10.1158/0008-5472.CAN-04-1800. [DOI] [PubMed] [Google Scholar]
- 79.Muralidharan-Chari V, Clancy J, Plou C, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19:1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henderson MC, Azorsa DO. The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol. 2012;2:38. doi: 10.3389/fonc.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 82.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 83.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 84.Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol. 2011;2011:1–11. doi: 10.1155/2011/842849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smyth TJ, Redzic JS, Graner MW, Anchordoqu TJ. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim Biophys Acta. 2014;1838:2954–2965. doi: 10.1016/j.bbamem.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fan TWM, Lane AN, Higashi RM, Bousamra M. Methods for detecting cancer. WO 2011/163332 A2 United States of America Patent Application. 2011
- 87.Smith ZJ, Lee C, Rojalin T, et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles. 2015;4:28533. doi: 10.3402/jev.v4.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gara S, Boussen H, Ghanem A, Guemira F. Use of common seric tumor markers in patients with solid cancers. Tunis Med. 2008;86:579–583. [PubMed] [Google Scholar]
- 89.Fernandis A, Wenk MR. Lipid-based biomarkers for cancer. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2830–2835. doi: 10.1016/j.jchromb.2009.06.015. [DOI] [PubMed] [Google Scholar]