Abstract
Purpose
Thrombomodulin (TM) is a multidomain, transmembrane protein with anti-inflammatory properties. Thrombomodulin domain (D) 1 is lectin-like, interacting with Lewis Y antigen on lipopolysaccharide, and with HMGB1, while TMD23 is associated with angiogenic and anti-inflammatory functions. Thus, we tested if TM is protective against Pseudomonas aeruginosa keratitis and whether it enhanced corneal vascularity.
Methods
Eyes of C57BL/6 (B6) mice were injected with recombinant TM (rTM), rTMD1, or PBS subconjunctivally before and intraperitoneally after infection with P. aeruginosa. Clinical scores, photography with a slit lamp, RT-PCR, ELISA, myeloperoxidase (MPO) assay, viable bacterial plate counts, and India ink perfusion were used to assess the disease response and corneal vascularity (rTM only).
Results
Recombinant TM versus PBS treatment reduced clinical scores and corneal opacity. Corneal mRNA levels for HMGB1 were unchanged, but proinflammatory molecules IL-1β, CXCL2, NF-κB, TLR4, and RAGE were decreased; anti-inflammatory molecules SIGIRR and ST2 were increased. ELISA confirmed the mRNA data for HMGB1, IL-1β, and CXCL2 proteins. Both neutrophil influx and viable bacterial plate counts also were decreased after rTM treatment. Protein levels for angiogenic molecules VEGF, VEGFR-1, and VEGFR-2 were measured at 5 days post infection and were not different or reduced significantly after rTM treatment. Further, perfusion with India ink revealed similar vessel ingrowth between the two groups. Similar studies were performed with rTMD1, but disease severity, mRNA, proteins, MPO, and plate counts were not changed from controls.
Conclusions
These data provide evidence that rTM treatment is protective against bacterial keratitis, does not reduce HMGB1, and is not angiogenic.
Keywords: thrombomodulin, Pseudomonas, treatment
Pseudomonas aeruginosa, a gram-negative bacterium, is a common opportunistic pathogen and a causative agent of microbial keratitis worldwide, resulting in severe ocular pain, corneal opacity, and reduced visual acuity.1 Traumatic ocular surface accidents in developing countries,2,3 extended wear soft contact lenses4, and preexisting long-standing ocular disease,5 are among the most prevalent risk factors. The disease is treated with antibiotics, but with emerging resistance to many commonly used antibacterials,6,7 alternative therapies are desirable.
Recently, this laboratory has shown that silencing or antibody neutralization of high mobility group box-1 (HMGB1) decreases P. aeruginosa–induced keratitis.8 HMGB1 is a mediator of the inflammatory response9,10 and is the most studied member of a family of molecules referred to as danger-associated molecular patterns or alarmins. Extracellular HMGB1 binds to cell surface inflammatory mediators such as Toll-like receptors (TLRs) and receptor for advanced glycation end products (RAGE) to initiate an inflammatory cascade.11,12 HMGB1 also contributes to pathogenesis in nonocular infectious10,13 and noninfectious diseases,14–17 and its reduction has been shown to be efficacious to disease outcome. Recently, a growing body of evidence suggests that the lectin-like domain of TM by reduction of HMGB1 may sequester its adverse effects,18,19 but the functional role of TM has not been tested in bacterial keratitis.
In this regard, TM is a multidomain transmembrane glycoprotein present in diverse vertebrate cells.20–25 It was identified as an anticoagulant factor that activates protein C,22 but recent reports23–25 suggest that TM is involved in biological processes in addition to hemostasis, including cell–cell adhesion, epithelial–mesenchymal transition, and inflammation. Soluble domains of TM include the N-terminal lectin-like domain (TMD1), the epidermal growth factor–like domain (TMD2), and the serine/threonine rich domain (TMD3).26 TMD1 mediates its anti-inflammatory role via two binding partners, the Lewis Y (Ley) antigen on lipopolysaccharide (LPS) and HMGB1. Ley–TMD1 interaction blocks LPS binding to CD14 and dampens the downstream inflammatory signaling cascade.27–29 Controversy exists regarding the role of TMD23 (composed of TMD2 and TMD3). One group30 has reported it to be angiogenic, but work from another laboratory31 does not support that conclusion. Furthermore, recently it has been shown that TMD23 is anti-inflammatory,32 binding to CD14 and inhibiting the CD14-mediated inflammatory response.
The current study examined the anti-inflammatory role of TM in P. aeruginosa bacterial keratitis and tested whether it was angiogenic. Our data provided evidence that treatment with recombinant TM (rTM) results in protection against keratitis in B6 mice, using a cytotoxic strain of P. aeruginosa. Recombinant TM treatment downregulated proinflammatory and upregulated anti-inflammatory cytokines at the mRNA level. The IL-1β and CXCL2 protein levels also were significantly lower in the rTM- than PBS-treated infected cornea and correlated with a decreased neutrophil influx. In addition, vascular endothelial growth factor (VEGF) angiogenic proteins at 5 days post infection (p.i.) were unchanged or decreased after rTM versus PBS treatment; similar vessel ingrowth was substantiated by India ink perfusion and quantification. Treatment with rTM also delayed disease in B6 mice infected with a noncytotoxic clinical isolate. Collectively, this study provided evidence that rTM is protective, not angiogenic, and may have therapeutic value in treating P. aeruginosa bacterial keratitis.
Materials and Methods
Mice
Eight-week-old female C57BL/6 (B6) mice (purchased from the Jackson Laboratory, Bar Harbor, ME, USA) were housed in accordance with the National Institutes of Health guidelines. Animals were treated humanely and in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Bacterial Culture and Infection
P. aeruginosa strain 19660 (American Type Culture Collection, Manassas, VA, USA) and KEI 1025 clinical isolate (Kresge Eye Institute, Detroit, MI, USA) were grown in peptone tryptic soy broth medium in a rotary shaker water bath at 37°C, 150 rpm for 18 hours to an optical density (measured at 540 nm) between 1.3 and 1.8. Bacterial cultures were pelleted by centrifugation at 5500g for 10 minutes. Pellets were washed once with sterile saline, recentrifuged, resuspended, and diluted in sterile saline to a final concentration of 1 × 106 or 1 × 107 CFU/μL. Anesthetized (using anhydrous ethyl ether) mice were placed beneath a stereoscopic microscope at ×40 magnification. The left cornea was scarified by making three 1-mm incisions with a sterile 255/8-gauge needle. The wounded corneal surface was topically treated with a 5 μL aliquot containing 1 × 106 CFU/μL (19660) or 1 × 107 CFU/μL (KEI 1025) of the P. aeruginosa suspension.
Ocular Response to Bacterial Infection
An established corneal disease grading scale33 was used to assign a clinical score value to each infected eye at 1, 3, and 5 days p.i. Clinical scores were used to statistically compare disease severity and were designated as follows: 0 = clear or slight opacity, partially or fully covering the pupil; +1 = slight opacity, fully covering the anterior segment; +2 = dense opacity, partially or fully covering the pupil; +3 = dense opacity, covering the entire anterior segment; and +4 = corneal perforation or phthisis. Photographs using a slit lamp were taken at 5 days p.i. to confirm and illustrate disease.
Recombinant TM Treatment
The left eyes of B6 mice (n = 5/group/time) were injected with 1 μg/5 μL rTM protein (mouse myeloma cell line NSO-derived, TM domains 1-4, Leu 17–Ser 517; R&D Systems, Minneapolis, MN, USA) or 5 μL PBS subconjunctivally, 1 day before infection. In separate selected experiments (using the cytotoxic strain), similar treatment was done by using rTMD1 (Biologics International Corp., Indianapolis, IN, USA). All mice were injected intraperitoneally on 1 and 3 days p.i. with 1 μg/100 μL rTM or rTMD1 protein (1 μg/100 μL) or 100 μL PBS.
Real-Time RT-PCR
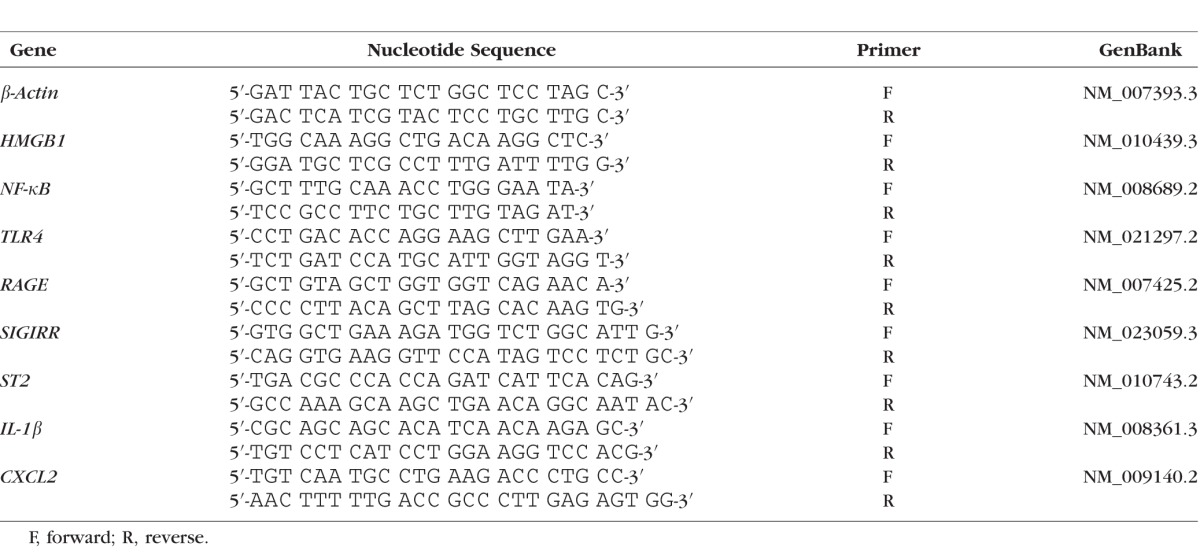
Recombinant TM–, rTMD1-, and PBS-treated B6 mice were killed at 5 days p.i. and the normal (uninfected) and infected (cytotoxic strain) corneas were harvested. For each individual cornea, total corneal RNA was isolated (RNA STAT-60; Tel-Test, Friendswood, TX, USA) according to the manufacturer's instructions. Upon spectrophotometric quantification at 260 nm, 1 μg of each RNA sample was reverse transcribed by using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA, USA) to produce a cDNA template for the PCR reaction. The cDNA products were diluted 1:25 with diethylpyrocarbonate-treated water. A 2 μL aliquot of diluted cDNA was used for the real-time RT-PCR reaction with Real-Time SYBR Green/Fluorescein PCR Master Mix (Bio-Rad, Richmond, CA, USA) and primer concentrations of 10 μM (total 10 μL reaction volume). After a preprogrammed hot start cycle (3 min. at 95°C), the parameters used for PCR amplification were 15 seconds at 95°C and 60 seconds at 60°C with the cycles repeated 45 times. Optimal conditions for PCR amplification of cDNA were established by using routine methods. The mRNA levels of HMGB1, NF-κB, TLR4, IL-1β, CXCL2, RAGE, single Ig IL-1–related receptor (SIGIRR), and interleukin 1 receptor-like 1 (ST2; IL-33 receptor) were tested by real-time RT-PCR (CFX Connect Real-Time PCR Detection System; Bio-Rad). The fold differences in gene expression were calculated after normalization to β-actin and are expressed as the relative mRNA concentration ± the standard error of the mean. The primer pair sequences used for real-time RT-PCR are shown in the Table.
Table.
Nucleotide Sequence of the Specific Primers Used for PCR Amplification
Enzyme-Linked Immunosorbent Assay
Recombinant TM-, rTMD1-, and PBS-treated B6 mice (5 mice/group/time) were killed at 3 and 5 days p.i. and the normal and infected (cytotoxic strain) corneas harvested. Individual corneas were homogenized in 500 μL PBS with 0.1% Tween 20 with a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) and centrifuged at 12,000g for 5 minutes. A 50 μL aliquot of each supernatant was assayed in duplicate to quantify IL-1β and CXCL2 proteins. For rTM-treated mice infected with the cytotoxic strain, a 100 μL aliquot of supernatant was assayed in duplicate to quantify HMGB1, VEGF, VEGF receptor 1 (VEGFR-1), and VEGF receptor 2 (VEGFR-2) proteins. ELISA kits were purchased from R&D Systems or from Chondrex, Inc. (Redmond, WA, USA) and assays were run by following the manufacturer's instructions. Sensitivities of the assays were as follows: 1.6 ng/mL (HMGB1), 2.31 pg/mL (IL-1β), 1.5 pg/mL (CXCL2), 9 pg/mL (VEGF), 13.3 pg/mL (VEGFR-1), and 11.4 pg/mL (VEGFR-2).
Myeloperoxidase Assay
A myeloperoxidase (MPO) assay was used to quantitate neutrophil cell number in the cornea of rTM-, rTMD1-, and PBS-treated mice infected with the cytotoxic strain. Individual corneas were removed at 3 and 5 days p.i. and homogenized in 1.0 mL of 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethyl-ammonium (Sigma-Aldrich Corp., St. Louis, MO, USA). Samples were freeze-thawed four times and after centrifugation, 100 μL supernatant was added to 2.9 mL of 50 mM phosphate buffer containing o-dianisidine dihydrochloride (16.7 mg/mL; Sigma-Aldrich Corp.) and hydrogen peroxide (0.0005%). The change in absorbency was monitored at 460 nm for 5 minutes at 30-second intervals. The slope of the line was determined for each sample and used to calculate units of MPO/cornea. One unit of MPO activity is equivalent to ∼2 × 105 neutrophils.34
Quantification of Viable Bacteria
Upon killing mice on 3 and 5 days p.i., infected corneas (using cytotoxic strain) were harvested from B6 mice (n = 5/group/time) treated with rTM, rTMD1, or PBS. Each cornea was homogenized in 1 mL sterile saline containing 0.25% BSA. A 100 μL aliquot of the corneal homogenate was serially diluted 1:10 in sterile saline containing 0.25% BSA. Selected dilutions were plated in triplicate on Pseudomonas isolation agar plates (Becton-Dickinson, Franklin Lakes, NJ, USA). Plates were incubated overnight at 37°C and the number of bacterial colonies manually counted. Results are reported as log10 CFU/cornea ± SEM.
India Ink Perfusion and Vessel Measurement
India ink perfusion was modified from a previous protocol35 and as reported before.36 Briefly, on 5 days p.i. with the cytotoxic strain, B6 mice treated with rTM or PBS were anesthetized with Avertin (Sigma-Aldrich Corp.), the thoracic cavity was opened, and the right atrium was incised. A 25-gauge winged infant infusion needle (Kawasumi Laboratories America, Tampa, FL, USA) was inserted into the left ventricle and mice were perfused with 8 mL PBS containing 72 U heparin by using a syringe-pump (Braintree Scientific, Braintree, MA, USA) set to deliver 90 mL/h. After, a second syringe containing Higgins black India ink was connected to the needle and 1 to 2 mL delivered. After killing mice, eyes (four quadrants) were photographed by using a slit lamp, and blood vessel ingrowth was measured in pixels with Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). Pixels were converted to micrometers by using a scale bar photographed at the same magnification. Three vessels were measured (from limbus to termination of vessels in the peripheral cornea) for each quadrant from each slit lamp photograph (n = 7 eyes/4 quadrants per eye/group/time).
Construction of rTMD1 Plasmid, Expression and Purification
Murine TMD1 cDNA was PCR amplified with SacI and SalI restriction sites from a murine lung cDNA template, and primers 5′-GCCGGATCCGCCAAGCTGCAGCC-3′ and 5′-CGCGAAGCTTTCAGAGGCCTGCAGG-3′ were used for the PCR amplification. The TMD1 PCR product and the pQE80L-Kan vector (Qiagen, Inc., Valencia, CA, USA) were digested with restriction enzymes SacI and SalI. Corresponding fragments were extracted by agarose gel electrophoresis and the TMD1 cDNA fragment was ligated to the pQE80L-Kan fragment by T4 DNA ligase. Then, the region between the His tag and the 5′ end of the TMD1-produced plasmid was deleted by Q5 site-directed mutagenesis with the following primers: 5′-GCCAAGCTGCAGCCCACA-3′ and 5′-GTGATGGTGATGGTGATGCGATC-3′ (New England Bio Labs, Ipswich, MA, USA). The final expression vector with the 5′ end of TMD1 fused to the His tag was confirmed by sequencing the construct (pTMD1). Expression of pTMD1 in Escherichia coli and His tag affinity purification of rTMD1 protein (>95% pure and <1 EU (endotoxin unit)/μg) was done by Biologics International Corp. (Indianapolis, IN, USA) and purchased for use in this study.
Statistical Analysis
The difference in clinical score between two groups at each time was tested by the Mann-Whitney U test. An unpaired, two-tailed Student's t-test was used to determine the statistical significance of the real-time RT-PCR, ELISA, MPO, and viable bacterial plate count data and was considered significant at P < 0.05. All experiments were repeated once to ensure reproducibility and unless stated otherwise, data from a representative experiment are shown as mean ± SEM.
Results
Recombinant TM Treatment Reduces Corneal Disease After P. aeruginosa Infection With Strain 19660
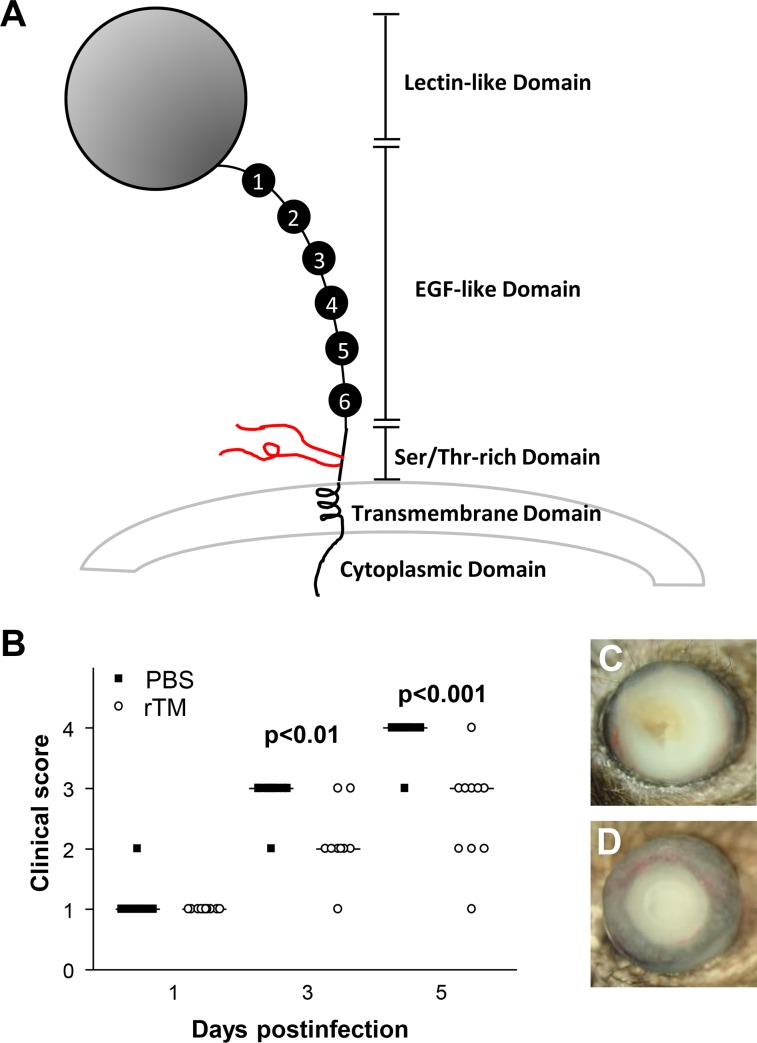
To test the effect of TM (shown diagrammatically in Fig. 1A) on host resistance to infection with a cytotoxic strain (19660) of P. aeruginosa, B6 mice were subconjunctivally and intraperitoneally injected with soluble rTM protein or PBS (control). Clinical scores (Fig. 1B) showed that rTM-treated mice exhibited reduced disease scores, significant at 3 and 5 days p.i. (P < 0.01 and P < 0.001), but not at 1 day p.i. Photographs taken with a slit lamp of P. aeruginosa–infected corneas of B6 mice treated with PBS (Fig. 1C) or rTM protein (Fig. 1D) showed that rTM-treated mice exhibited less severe disease/corneal opacity than that of the PBS control.
Figure 1.
(A–D) Thrombomodulin domains. Thrombomodulin (A) is shown diagrammatically to illustrate its multidomain (5) composition. The rTM used in this study consisted of amino acids 17 to 517, corresponding to the extracellular portion (all domains except cytoplasmic) of the type I membrane protein. Significantly different clinical scores (B) were seen at 3 and 5 days p.i. in rTM- as compared with PBS-treated mice. Photographs taken with a slit lamp at 5 days p.i. showed less opacity/disease after rTM (C) compared with PBS treatment (D). Magnification (C, D) = ×3.5.
Effect of TM on Expression of Pro- and Anti-Inflammatory Genes
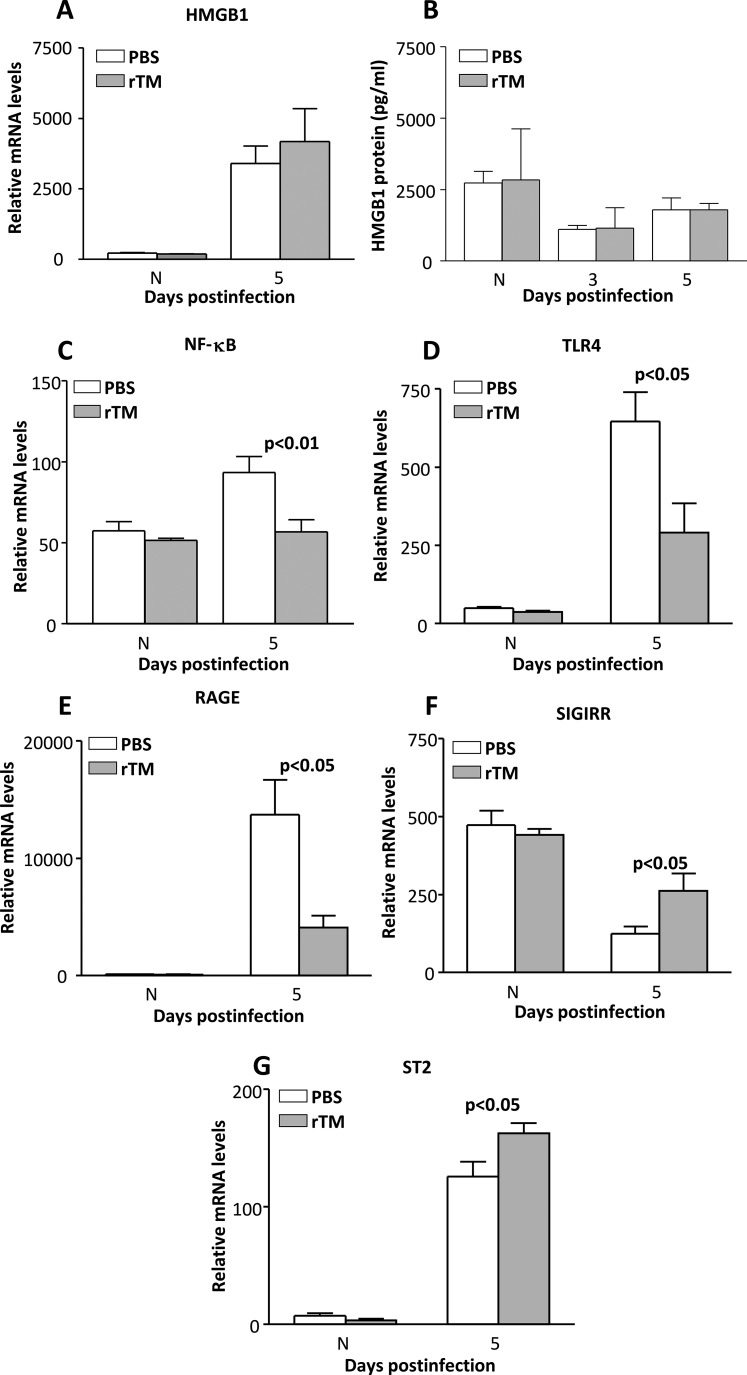
The HMGB1 mRNA (Fig. 2A) levels were slightly but not significantly elevated after rTM treatment; no difference was seen in the normal cornea between groups. Figure 2B shows ELISA analysis of HMGB1 protein levels, which were not reduced by rTM treatment and did not differ between groups at either period tested.
Figure 2.
(A–G) Recombinant TM treatment of B6 mice. After rTM treatment, relative mRNA (A) or protein (B) levels of HMGB1 did not differ between groups at 3 and/or 5 days p.i. Messenger RNA expression of NF-κB (C), TLR4 (D), and RAGE (E) were significantly decreased in rTM compared with PBS controls at 5 days p.i. In contrast, levels of SIGIRR (F) and ST2 (G) were significantly elevated. No difference between groups was seen for normal (N) cornea.
Relative corneal mRNA levels of additional pro- and anti-inflammatory molecules after rTM versus PBS treatment were tested (Figs. 2C–G). Among the proinflammatory genes tested, relative mRNA levels were significantly downregulated in the cornea of rTM-treated mice at 5 days p.i. The genes with decreased mRNA levels include NF-κB (Fig. 2C, P < 0.01), TLR4 (Fig. 2D, P < 0.05), and RAGE (Fig. 2E, P < 0.05). Relative mRNA levels for anti-inflammatory genes SIGIRR (Fig. 2F) and ST2 (Fig. 2G) were upregulated in infected corneas of rTM- versus PBS-treated mice at 5 days p.i. (P < 0.05 for each). There were no differences between mRNA levels (or protein) of the molecules tested for any of the normal uninfected mouse corneas between PBS and rTM groups (Figs. 2A–G).
Recombinant TM Treatment Led to a Reduced Inflammatory Environment
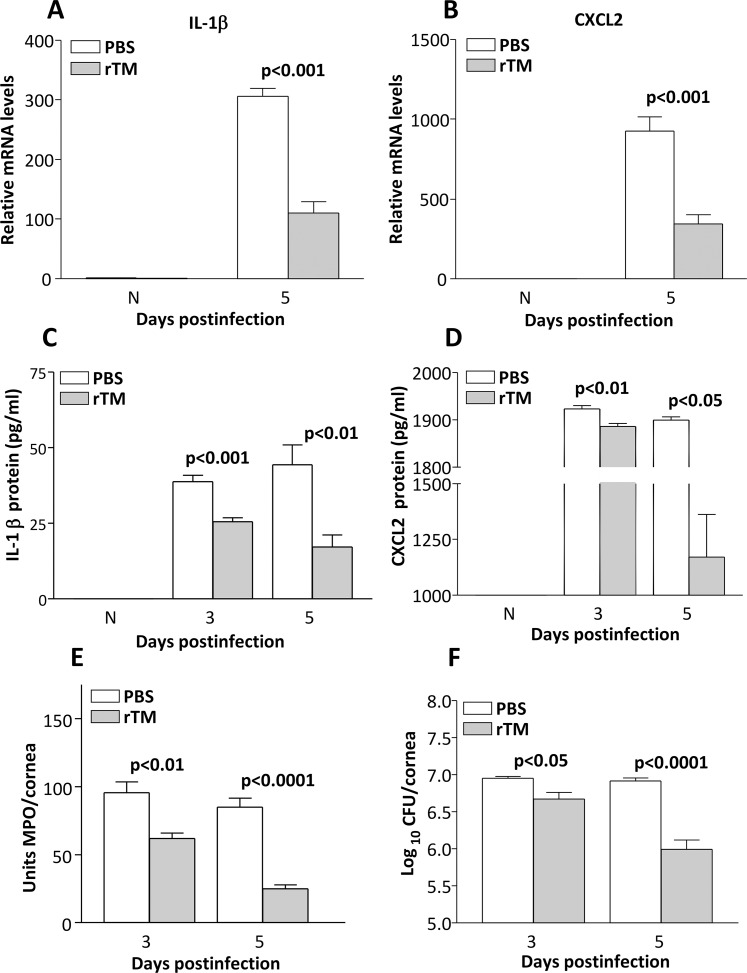
Messenger RNA levels of IL-1β (Fig. 3A, P < 0.001) and CXCL2 (Fig. 3B, P < 0.001) were decreased after infection following rTM treatment. In the uninfected normal cornea, these proteins were below detectability for both groups (Figs. 3A, 3B). Recombinant TM treatment significantly decreased expression of IL-1β protein in the P. aeruginosa–infected corneas at both 3 and 5 days p.i. (Fig. 3C, P < 0.001 and P < 0.01). Expression of CXCL2 protein also was decreased at 3 (P < 0.01) and 5 (P < 0.05) days p.i. (Fig. 3D). Since rTM treatment decreased protein levels of these two cytokines known to regulate neutrophil influx, an MPO assay was used to quantitate the cell infiltrate in the corneas of rTM- and PBS-treated B6 mice at 3 and 5 days p.i. The MPO activity was significantly reduced both at 3 (P < 0.01) and 5 (P < 0.0001) days p.i. in rTM- versus PBS-treated mice (Fig. 3E). Bacterial plate counts (Fig. 3F) were used to detect viable bacteria in the infected cornea of mice treated with rTM versus PBS at 3 and 5 days p.i. Recombinant TM treatment led to decreased bacterial load at 3 (P < 0.05) and 5 (P < 0.0001) days p.i., compared to controls.
Figure 3.
(A–F) Recombinant TM treatment of B6 mice. The IL-1β mRNA (A) and protein (C) and CXCL2 mRNA (B) and protein (D) levels were significantly reduced at 3 and/or 5 days p.i. after rTM. No difference in mRNA or protein between groups was detected in the N cornea. The MPO levels (E) also were reduced significantly at 3 and 5 days p.i. Viable bacterial plate counts were reduced at both 3 and 5 days p.i. after rTM (F).
Recombinant TM Treatment Is Not Angiogenic
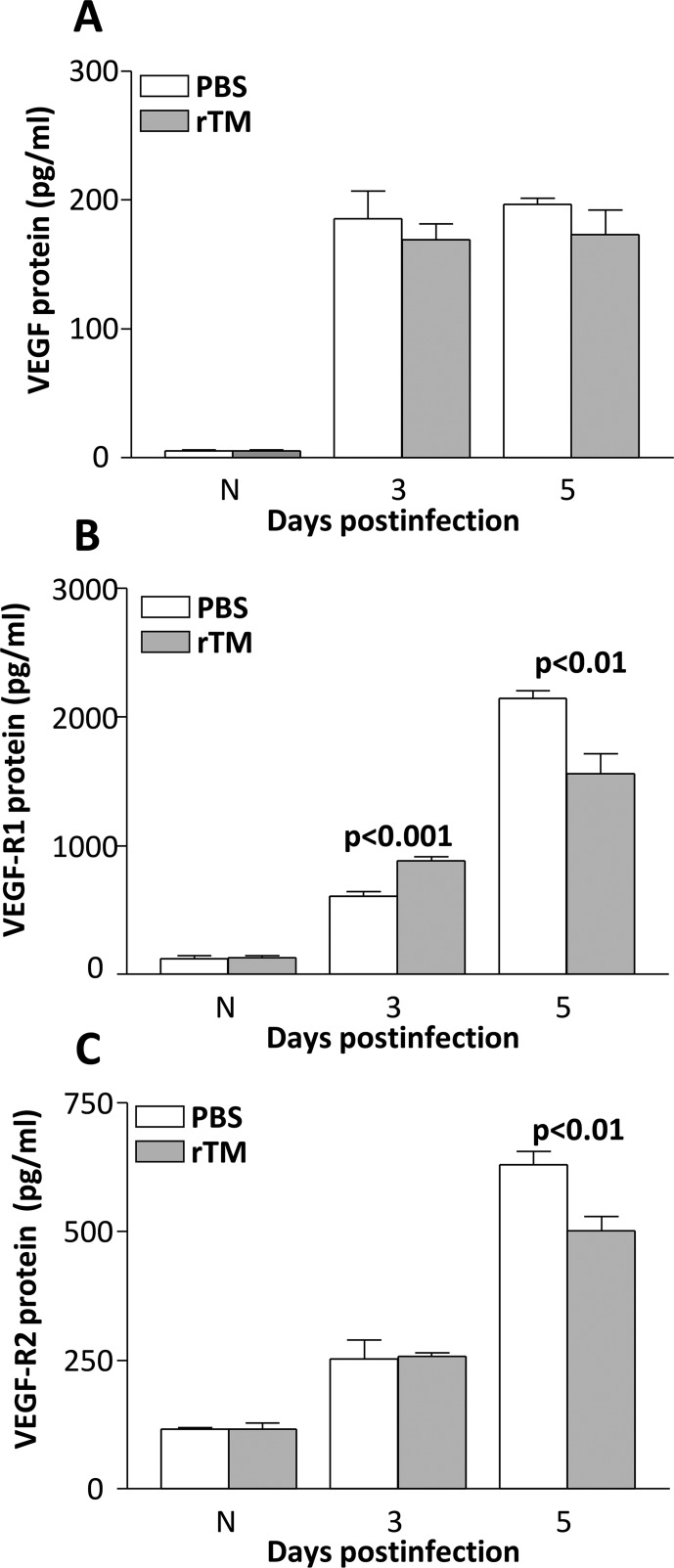
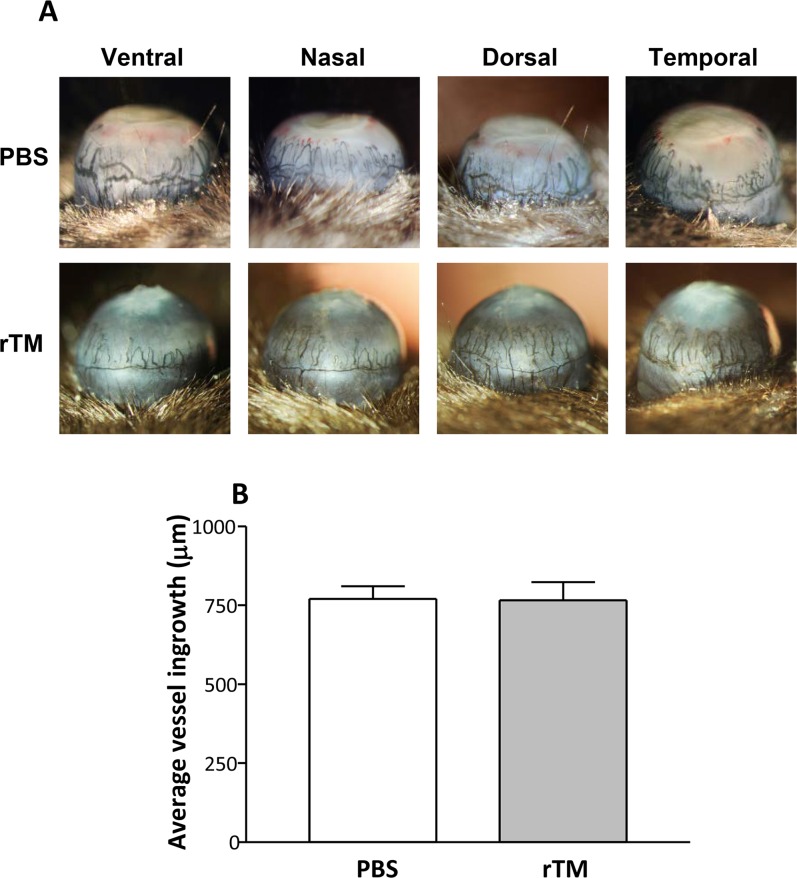
To test if TMD23, two domains within rTM, had angiogenic properties, expression patterns for common angiogenic molecules were assessed after rTM versus PBS treatment at 3 and 5 days p.i. (Figs. 4A–C). At 3 days p.i., rTM versus PBS treatment led to a slight but not significant decrease in VEGF (Fig. 4A), a modest, but significant increase in VEGFR-1 (Fig. 4B, P < 0.001), and no difference in VEGFR-2 protein expression (Fig. 4C). The VEGFR-1 and VEGFR-2 protein expression was significantly downregulated (Figs. 4B, 4C) in the rTM- versus PBS-treated group at 5 days p.i. (P < 0.01). The VEGF protein expression also was downregulated compared to control levels at 5 days p.i., but was not significant (Fig. 4A). No differences in protein levels were detected for the uninfected corneas of rTM- versus PBS-treated mice, for all three angiogenic molecules tested. To further test angiogenesis, an India ink perfusion experiment (Fig. 5) was used to determine if the protein expression profile of angiogenic molecules at 5 days p.i. correlated with vessel ingrowth in rTM- versus PBS-treated, infected mice. Photographs with a slit lamp (Fig. 5A) allowed visualization of blood vessel ingrowth in the eyes of both treatment groups in the ventral, nasal, dorsal, and temporal planes. Statistical analysis of averaged values of vessel ingrowth (Fig. 5B) revealed no differences between groups and confirmed the visual observations after India ink perfusion.
Figure 4.
(A–C) Angiogenic molecules. Protein levels for VEGF (A) did not differ between groups after rTM treatment (both times tested). For VEGFR-1 (B), levels were increased at 3 but decreased at 5 days p.i after rTM treatment. For VEGFR-2 (C), no difference between groups was seen at 3 days p.i., but levels were decreased with rTM treatment at 5 days p.i. No difference between groups was seen for the N cornea.
Figure 5.
(A, B) India ink perfusion of infected eyes at 5 days p.i. (A) Recombinant TM– and PBS-treated eyes were observed and photographed in four planes (ventral, nasal, dorsal, and temporal) and the average vessel ingrowth measured. No significant difference was detected between groups (B). Magnification (A) = ×5 for all photographs.
Recombinant TMD1 Treatment: Clinical Score, Slit Lamp Photography, and mRNA Levels of Pro- and Anti-Inflammatory Cytokines
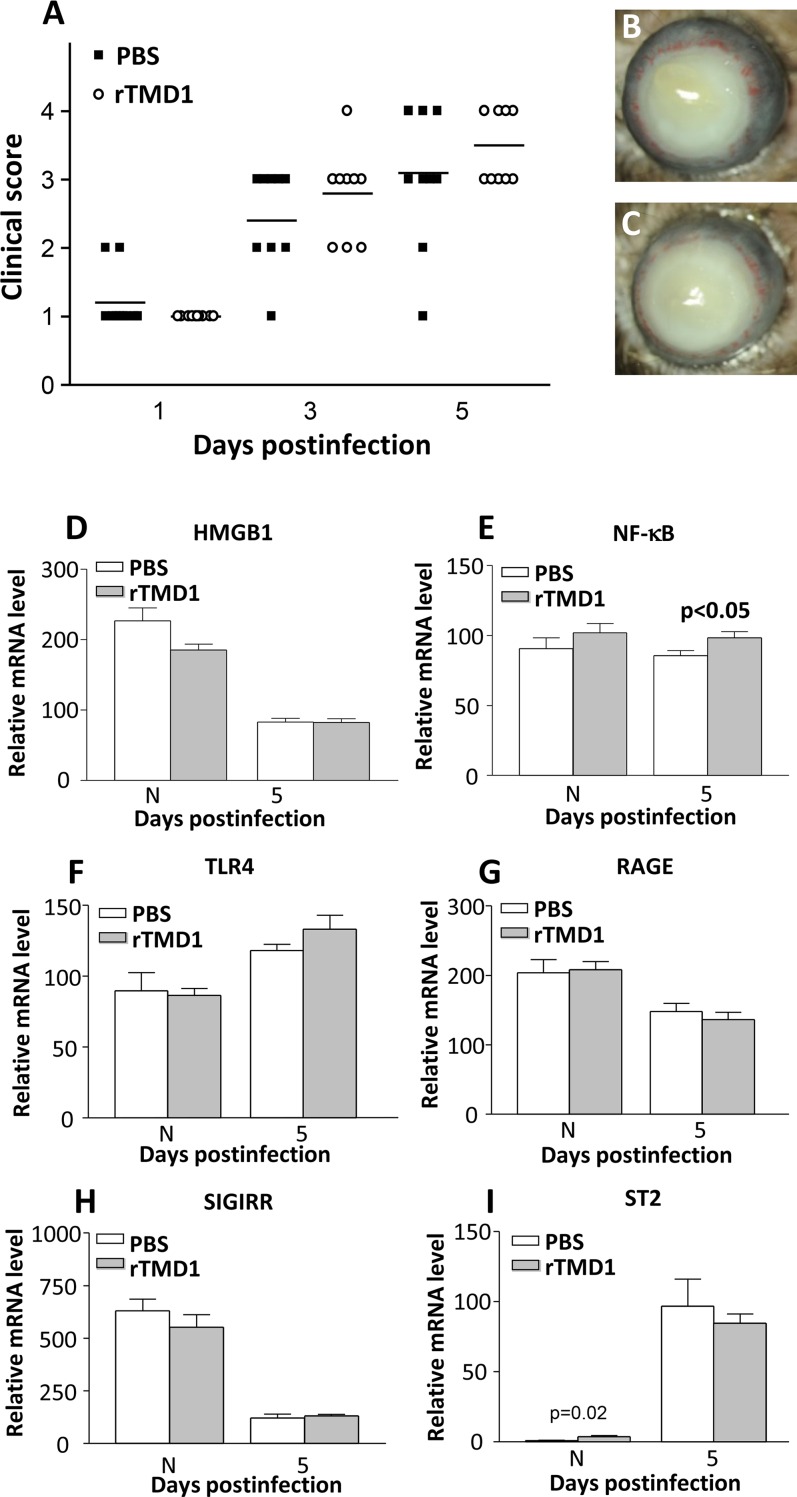
To test whether the lectin-like domain of TM was responsible for the protective effects that were seen with rTM treatment using strain 19660, similar experiments were performed by using rTMD1 protein. Clinical scores (Fig. 6A) showed that rTMD1-treated mice exhibited no significant differences between groups at any period tested. Figures 6B and 6C are photographs taken with a slit lamp of infected corneas of B6 mice treated with PBS (Fig. 6B) or rTMD1 protein (Fig. 6C), respectively, at 5 days p.i. Corneas of rTMD- treated mice exhibited no difference in disease severity as compared to PBS controls. Figure 6D and Figures 6F through 6I confirmed this, as no significant changes in mRNA levels between treatment groups for HMGB1 (Fig. 6D), TLR4 (Fig. 6F), RAGE (Fig. 6G), SIGIRR (Fig. 6H), or ST2 (Fig. 6I) were seen. The NF-κB (Fig. 6E) levels were slightly but significantly (P < 0.05) increased at 5 days p.i. No differences were observed in normal corneas between groups, except for ST2, which was slightly but significantly (P = 0.02) higher after rTMD1 treatment.
Figure 6.
(A–I) Recombinant TMD1 treatment of B6 mice. No significant different clinical scores (A) were seen at any time point tested between rTMD1- compared with PBS-treated mice. Photographs taken with a slit lamp at 5 days p.i. showed similar opacity in rTMD1-treated (C) and PBS-treated (B) eyes. After rTMD1 treatment, relative mRNA for HMGB1 (D), TLR4 (F), and RAGE (G) did not differ in rTM compared with PBS controls at 5 days p.i.; NF-kB (E) levels were elevated (P < 0.05). Neither did levels of SIGIRR (H) or ST2 (I) differ between groups. No differences between groups was seen for N cornea except for ST2 (P = 0.02). Magnification (B, C) = ×5.
Recombinant TMD1 Treatment: Protein, MPO, and Plate Counts
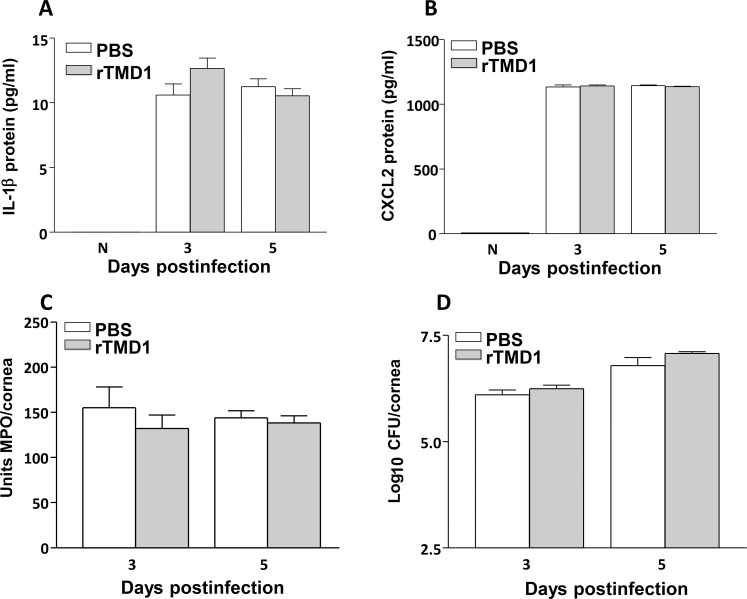
To confirm the mRNA data, protein levels were selectively tested. The rTMD1 treatment did not result in differences between groups for IL-1β (Fig. 7A), or for CXCL2 protein (Fig. 7B) at 5 days p.i. No differences between groups were seen for the normal cornea. An MPO assay confirmed that there was no difference in the neutrophil infiltrate between groups (Fig. 7C) at either time tested. Bacterial plate counts (Fig. 7D) also did not differ between groups at similar times.
Figure 7.
(A–D) Recombinant TMD1 treatment of B6 mice. The IL-1β (A) and CXCL2 (B) protein levels did not differ at 3 and/or 5 days p.i. or in the N cornea between groups. Neither MPO levels (C) nor viable bacterial plate counts (D) were reduced after rTMD1.
Recombinant TM Treatment Following Infection With Strain KEI 1025, a Clinical Isolate
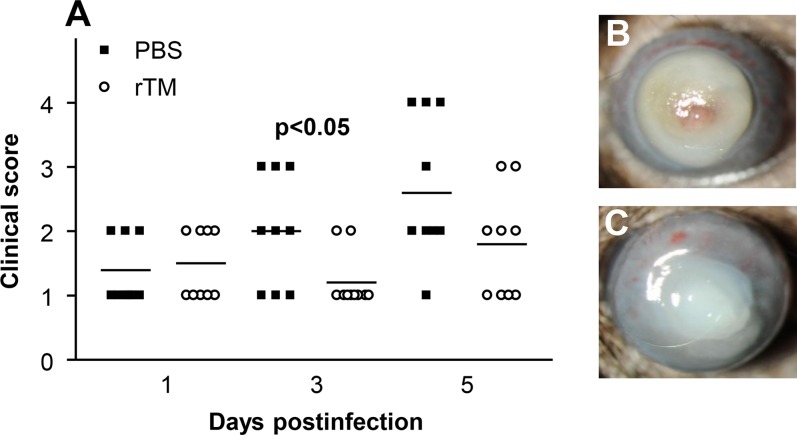
We also tested the efficacy of rTM by using a noncytotoxic clinical isolate. Clinical scores (Fig. 8A) showed a significant difference (P < 0.05) between groups only at 3 days p.i. However, at 5 days p.i., no corneas in the rTM-treated compared to the PBS control group had perforated. Photographs with a slit lamp confirmed the clinical score data and showed perforation in the PBS-treated group (Fig. 8B); no perforation was observed in the rTM treatment group (Fig. 8C).
Figure 8.
(A–C) Recombinant TM and infection with clinical isolate. Significantly different clinical scores (A) were seen at 3 but not 1 or 5 days p.i. in rTM- compared with PBS-treated mice infected with KEI 1025. Photographs taken with a slit lamp at 5 days p.i. showed similar opacity/disease after rTM (C) compared with PBS (B) treatment. Magnification (B, C) = ×6.5.
Discussion
Thrombomodulin is a transmembrane glycoprotein identified originally in the vascular endothelium.21 The molecule is composed of five domains: a highly charged N-terminal lectin-like domain (TMD1), a domain with six epidermal growth factor (EGF)–like structures (TMD2), a serine and threonine–rich domain (TMD3), a transmembrane domain (TMD4), and a cytoplasmic domain (TMD5).24,28 It is a multifunctional protein and has distinct functions dependent on cell type. For example, in keratinocytes, TM expression is regulated during differentiation37 and in cutaneous wound healing, release of soluble TM from these cells appears to promote wound closure.38 In this regard, recombinant TM EGF-like domain plus a serine/threonine-rich domain, rTMD23, promote cutaneous healing in a mouse wound healing model39 and in corneal epithelial wound repair.31 In addition, the role of TM has been studied in inflammatory diseases in the eye, including endotoxin-induced uveitis.29 Results show that in these diseases, TM expression is observed in the corneal epithelium and in stromal cells. In addition, the distribution of TM is similar in the eye of humans20 and mice,31 suggesting a potential for similarity of TM function between the two groups.
In this study we provided evidence that treatment of experimental bacterial keratitis with rTM, composed of domains TMD1-TMD4 (Leu 17–Ser 517), is protective against perforation in a mouse model of bacterial keratitis. Protection appeared to correlate with mechanisms of virulence, in that better protection was achieved for a cytotoxic (19660) versus a noncytotoxic clinical isolate. For the cytotoxic strain, which was tested in detail, treatment with rTM resulted in reduction of proinflammatory molecules, including IL-1β and CXCL2, as well as a reduction in MPO activity consistent with reducing the two cytokines that have been shown to be associated with the cellular infiltrate in keratitis, which is mainly neutrophilic.40 Also consistent with fewer neutrophils in the stroma, which would reduce bystander damage and decrease nutrition for bacterial growth, the viable plate count also was reduced. It is also worth considering that other factors not tested herein could play a role in this outcome. In this regard, it has been shown that human TM regulates complement activation in response to xenogeneic stimuli41; that elevated plasma concentrations of nitric oxide correlates with elevated soluble TM levels in lupus patients42; that in a mutation of TM, activation of protein C is impaired, leading to increased neutrophils and inflammation in melioidosis, although it has no impact on bacterial growth or dissemination43; and that its lectin-like domain binding to Lewis Y antigen could neutralize LPS-induced inflammation.27 In contrast, we had expected to see reduction of HMGB1, which would in turn reduce rather than amplify inflammation, as shown in other systems8,13–17; however, the molecule was not reduced at either the mRNA or protein level. Based on this, we hypothesized that reduction in keratitis could be due to the possibility that the rTM that we used, which included domain 23, may have had an angiogenic effect, as has been shown by others,30 and that this might play a role in the healing response we observed. Alternatively, because the rTM that we used did not reduce HMGB1, we hypothesized that this could be because it lacked the full TMD1 lectin-like domain, which binds HMGB1,18 interferes with its binding to RAGE, impairing HMGB1-RAGE signaling18 or enhancing thrombin-mediated proteolytic degradation of HMGB1.19
First we focused on the function of the TMD23 portion of rTM used for treatment. Previously, domain 23 has been considered an angiogenic factor,30 promoting mouse cutaneous wound healing through modulating angiogenesis at the wound site.38,39 Thus, to determine whether the anti-inflammatory effects we observed were because of a possible angiogenic effect of rTM containing domains 23, classic angiogenic molecule VEGF, and its R1 and R2 receptor proteins, were measured in the infected cornea in rTM-treated and control mice. The VEGF protein did not differ at either 3 or 5 days p.i., while the level of R1 was slightly elevated at 3 and reduced at 5 days; R2 levels were unchanged at 3 and reduced at 5 days p.i. after rTM treatment. Because numerous molecules are angiogenic and it would be impossible to test all of them, India ink35,36 was used to globally assess the angiogenic potential of the 23 domain contained in rTM. Visual, followed by statistical analysis of these data showed no difference between treatment groups and did not support an angiogenic effect for rTM treatment. These results are consistent with those of Huang et al.,31 who do not report an angiogenic effect for the 23 domain of TM in corneal wound healing in vivo and in vitro. Huang et al.31 have further suggested that rTMD23 promotes corneal epithelial wound healing by accelerating epithelial cell migration and proliferation in the absence of corneal neovascularization. This is consistent with other work showing that both cell migration and proliferation play important roles during corneal epithelial wound healing,44,45 that rTMD23 has mitogenic activity for Swiss 3T3 cells46 and enhances proliferation and migration in endothelial cells.44 These data support our study showing no detectable angiogenic effect for rTM (containing the 23 domain of TM), although we did not directly test for epithelial cell migration and proliferation, as we were mainly concerned about the potential of an angiogenic effect. In fact, others32 have shown that rTMD23 has an anti-inflammatory effect by markedly suppressing the activation of intracellular signaling pathways and the production of inflammatory cytokines induced by LPS. They also have found that rTMD23 interacts with the soluble and membrane forms of CD14 and inhibits the CD14-mediated inflammatory response.32 These data are consistent with our own showing reduction of TLR4, albeit at the mRNA level, but most importantly with reduction in IL-1β and CXCL2 proteins, which are downstream effectors of LPS signaling.
Another anti-inflammatory target of TM is HMGB1, a ubiquitously expressed nuclear protein that is released from necrotic cells. Upon being released, HMGB1 binds to RAGE.47 HMGB1-RAGE signaling has been implicated in the pathogenesis and/or progression of various clinical disorders, such as infections, sepsis, arthritis, and cancer.47 Specifically, the lectin-like domain of TM interferes with HMGB1 binding to RAGE, thereby impairing HMGB1-RAGE signaling.18 In this regard, we have previously shown that reduction of HMGB1 levels via silencing or antibody treatment protects mice infected with P. aeruginosa and reduces disease.8 In the current study, rTM treatment lowered clinical scores, and slit lamp documented less disease/opacity in treated mice at 5 days after infection. However, examination of HMGB1, one of the targets of TM (domain 1), showed no differences in mRNA or protein levels between groups. In addition, rTM treatment lowered mRNA levels for several proinflammatory molecules including NF-κβ, TLR4, and RAGE while also providing a modest, yet significant shift in anti-inflammatory cytokines such as SIGIRR40 and ST2,48 which we have previously shown contribute to better disease outcome. TMD1 also mediates its anti-inflammatory role via binding the Ley antigen on LPS.27 Ley–TMD1 interaction blocks LPS binding to CD14 and TLRs and dampens the downstream inflammatory signaling cascade.28,29
Because the rTM that we purchased did not consist of a complete TMD1, we used rTMD1 protein and tested it after infection with the cytotoxic strain. The full TMD1 recombinant protein failed to modulate disease with similar outcome as controls for all pro- and anti-inflammatory molecules tested, which were the same as tested for rTM treatment. Neither were MPO or plate counts changed, suggesting that in keratitis, TMD1 alone is not capable of reducing disease.
Our data are also consistent with other studies using Recomodulin, a recombinant form of human soluble (rhs) TM, comprising the extracellular domain of TM that includes the N-terminal C-type lectin domain, EGF-like domain, and O-glycosylation domain.49 Administration of rhs-TM has been shown to protect rats from endotoxin-induced disseminated intravascular coagulation (DIC) or lung injury.50 In addition, rhs-TM not only reduces compression trauma-induced spinal cord injury by inhibiting leukocyte accumulation and expression of TNF-α51 but also provides protection against ischemia reperfusion injury in the canine liver52 and in the rat kidney.53,54 After obtaining promising results in animal experiments, rhs-TM (ART-123, Recomodulin) has proceeded to clinical trials.55 This clinical trial for DIC resulting from infection has shown that rhs-TM treatment improves the mortality rates at day 28 to a greater degree than does heparin treatment (rhs-TM 28.0%; heparin 34.6%).
In summary, treatment with rTM reduced inflammation and was protective in bacterial keratitis, using a cytotoxic strain of bacteria, and slowed disease progression, using a noncytotoxic clinical isolate. Reduction in inflammatory mediators such as IL-1β and CXCL2 led to decreased neutrophilic infiltrate and less stromal destruction, as well as decreased viable plate count. Recombinant TMD1 alone did not appear to have an anti-inflammatory effect in the keratitis model and was consistent with no observable reduction in HMGB1 levels following rTM treatment.
Acknowledgments
Supported by Grants R01EY002986, R01EY016058, and P30EY004068 from the National Eye Institute, National Institutes of Health and by a Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology, Kresge Eye Institute. LDH is the recipient of a 2012 Alcon Research Institute Award.
Disclosure: S.A. McClellan, None; S.A. Ekanayaka, None; C. Li, None; X. Jiang, None; R.P. Barrett, None; L.D. Hazlett, None
References
- 1. Pachigolla G,, Blomquist P,, Cavanagh, HD. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in north Texas. Eye Contact Lens. 2007; 33: 45–49. [DOI] [PubMed] [Google Scholar]
- 2. Bharathi MJ,, Ramakrishnan R,, Meenakshi R,, Padmavathy S,, Shivakumar C,, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007; 14: 61–69. [DOI] [PubMed] [Google Scholar]
- 3. Bharathi MJ,, Ramakrishnan R,, Meenakshi R,, Shivakumar C,, Raj DL. Analysis of the risk factors predisposing to fungal bacterial & Acanthamoeba keratitis in south India. Indian J Med Res. 2009; 130: 749–757. [PubMed] [Google Scholar]
- 4. Stapleton F,, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye. 2012; 26: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Neill EC,, Yeoh J,, Fabinyi DC,, et al. Risk factors, microbial profiles and prognosis of microbial keratitis-associated endophthalmitis in high-risk eyes. Graefes Arch Clin Exp Ophthalmol. 2014; 252: 1457–1462. [DOI] [PubMed] [Google Scholar]
- 6. Wong RL,, Gangwani RA,, Yu LW,, Lai JS. New treatments for bacterial keratitis. J Ophthalmol. 2012; 2012: 831502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breidenstein EBM,, de la Fuentes-Nunez C,, Hancock REW. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011; 19: 419–426. [DOI] [PubMed] [Google Scholar]
- 8. McClellan S,, Jiang X,, Barrett R,, Hazlett LD. High-mobility group box 1: a novel target for treatment of Pseudomonas aeruginosa keratitis. J Immunol. 2015; 194: 1776–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang H,, Bloom O,, Zhang M,, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999; 285: 248–251. [DOI] [PubMed] [Google Scholar]
- 10. Wang H,, Yang H,, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004; 255: 320–331. [DOI] [PubMed] [Google Scholar]
- 11. Andersson U,, Wang H,, Palmblad K,, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000; 192: 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andersson U,, Erlandsson-Harris H,, Yang H,, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol. 2002; 72: 1084–1091. [PubMed] [Google Scholar]
- 13. Sunden-Cullberg J,, Norrby-Teglund A,, Rouhiainen A,, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005; 33: 564–573. [DOI] [PubMed] [Google Scholar]
- 14. Pisetsky DS,, Erlandsson-Harris H,, Andersson U. High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther. 2008; 10: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Voll RE,, Urbonaviciute V,, Furnrohr B,, Herrmann M,, Kalden JR. The role of high-mobility group box 1 protein in the pathogenesis of autoimmune diseases. Curr Rheumatol Rep. 2008; 10: 341–342. [DOI] [PubMed] [Google Scholar]
- 16. Andersson U,, Harris HE. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys Acta. 2010; 1799: 141–148. [DOI] [PubMed] [Google Scholar]
- 17. Koulis C,, de Haan JB,, Allen TJ. Novel pathways and therapies in experimental diabetic atherosclerosis. Expert Rev Cardiovasc Ther. 2012; 10: 323–335. [DOI] [PubMed] [Google Scholar]
- 18. Abeyama K,, Stern DM,, Ito Y,, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005; 115: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ito T,, Kawahara K,, Okamoto K,, et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008; 28: 1825–1830. [DOI] [PubMed] [Google Scholar]
- 20. Ikeda T,, Ishii H,, Higuchi T,, et al. Localization of thrombomodulin in the anterior segment of the human eye. Invest Ophthalmol Vis Sci. 2000; 41: 3383–3390. [PubMed] [Google Scholar]
- 21. Maruyama I,, Bell CE,, Majerus PW. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985; 101: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raife TJ,, Lager DJ,, Madison KC,, et al. Thrombomodulin expression by human keratinocytes: induction of cofactor activity during epidermal differentiation. J Clin Invest. 1994; 93: 1846–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawanami O,, Jin E,, Ghazizadeh M,, et al. Heterogeneous distribution of thrombomodulin and von Willebrand factor in endothelial cells in the human pulmonary microvessels. J Nippon Med Sch. 2000; 67: 118–125. [DOI] [PubMed] [Google Scholar]
- 24. Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012; 34: 107–125. [DOI] [PubMed] [Google Scholar]
- 25. Yerkovich ST,, Roponen M,, Smith ME,, et al. Allergen-enhanced thrombomodulin (blood dendritic cell antigen 3, CD141) expression on dendritic cells is associated with a TH2-skewed immune response. J Allergy Clin Immunol. 2009; 123: 209–216. e204. [DOI] [PubMed] [Google Scholar]
- 26. Wang L,, Jiang R,, Sun XL. Recombinant thrombomodulin of different domains for pharmaceutical, biomedical, and cell transplantation applications. Med Res Rev. 2014; 34: 479–502. [DOI] [PubMed] [Google Scholar]
- 27. Shi CS,, Shi GY,, Hsiao HM,, et al. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood. 2008; 112: 3661–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li YH,, Kuo CH,, Shi GY,, Wu HL. The role of thrombomodulin lectin-like domain in inflammation. J Biomed Sci. 2012; 19: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jin H,, Yang X,, Liu K,, Gu Q,, Xu X. Effects of a novel peptide derived from human thrombomodulin on endotoxin-induced uveitis in vitro and in vivo. FEBS Lett. 2011; 585: 3457–3464. [DOI] [PubMed] [Google Scholar]
- 30. Shi CS,, Shi GY,, Chang YS,, et al. Evidence of human thrombomodulin domain as a novel angiogenic factor. Circulation. 2005; 111: 1627–1636. [DOI] [PubMed] [Google Scholar]
- 31. Huang YH,, I CC, Kuo CH,, et al. Thrombomodulin promotes corneal epithelial wound healing. PLoS One. 2015; 10: e0122491. [DOI] [PMC free article] [PubMed]
- 32. Ma CY,, Chang WE,, Shi GY,, et al. Recombinant thrombomodulin inhibits lipopolysaccharide-induced inflammatory response by blocking the functions of CD14. J Immunol. 2015; 194: 1905–1915. [DOI] [PubMed] [Google Scholar]
- 33. Hazlett LD,, Moon MM,, Strejc M,, Berk RS. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987; 28: 1978–1985. [PubMed] [Google Scholar]
- 34. Williams RN,, Paterson CA,, Eakins KE,, Bhattacherjee P. Quantification of ocular inflammation: evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Curr Eye Res. 1982; 2: 465–470. [DOI] [PubMed] [Google Scholar]
- 35. Zheng M,, Deshpande S,, Lee S,, Ferrara N,, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001; 75: 9828–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McClellan SA,, Wu M,, Zhang Y,, Barrett RP,, Hazlett LD. Toll-like receptor 4 regulation of corneal angiogenesis. Curr Angiogenesis. 2012; 1: 308–317. [Google Scholar]
- 37. Lager DJ,, Callaghan EJ,, Worth SF,, Raife TJ,, Lentz SR. Cellular localization of thrombomodulin in human epithelium and squamous malignancies. Am J Pathol. 1995; 146: 933–943. [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng TL,, Wu YT,, Lin HY,, et al. Functions of rhomboid family protease RHBDL2 and thrombomodulin in wound healing. J Invest Dermatol. 2011; 131: 2486–2494. [DOI] [PubMed] [Google Scholar]
- 39. Cheng TL,, Wu YT,, Lai CH,, et al. Thrombomodulin regulates keratinocyte differentiation and promotes wound healing. J Invest Dermatol. 2013; 133: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 40. Huang X,, Hazlett LD,, Du W,, Barrett RP. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J Immunol. 2006; 177: 548–556. [DOI] [PubMed] [Google Scholar]
- 41. Kim H,, Hawthorne WJ,, Kang HJ,, et al. Human thrombomodulin regulates complement activation as well as the coagulation cascade in xeno-immune response. Xenotransplantation. 2015; 22: 260–272. [DOI] [PubMed] [Google Scholar]
- 42. Ho CY,, Wong CK,, Li EK,, Tam LS,, Lam CWK. Elevated plasma concentrations of nitric oxide, soluble thrombomodulin and soluble vascular cell adhesion molecule-1 in patients with systemic lupus erythematosus. Rheumatology. 2003; 42: 117–122. [DOI] [PubMed] [Google Scholar]
- 43. Kager LM, JoostWiersinga W,, Roelofs JJTH,, et al. A thombomodulin mutation that impairs active protein C generation is detrimental in severe pneumonia-derived gram negative sepsis (Meliodosis). PLoS Negl Trop Dis. 2014; 8: e2819. [DOI] [PMC free article] [PubMed]
- 44. Agrawal VB,, Tsai RJ. Corneal epithelial wound healing. Indian J Ophthalmol. 2003; 51: 5–15. [PubMed] [Google Scholar]
- 45. Buck RC. Cell migration in repair of mouse corneal epithelium. Invest Ophthalmol Vis Sci. 1979; 18: 767–784. [PubMed] [Google Scholar]
- 46. Hamada H,, Ishii H,, Sakyo K,, Horie S,, Nishiki K,, Kazama M. The epidermal growth factor-like domain of recombinant human thrombomodulin exhibits mitogenic activity for Swiss 3T3 cells. Blood. 1995; 86: 225–233. [PubMed] [Google Scholar]
- 47. Lotze MT,, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005; 5: 331–342. [DOI] [PubMed] [Google Scholar]
- 48. Huang X,, Du W,, Barrett RP,, Hazlett LD. ST2 is essential for TH2 responsiveness and resistance to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007; 48: 4626–4633. [DOI] [PubMed] [Google Scholar]
- 49. Ito T,, Maruyama I. Thrombomodulin: protectorate God of the vasculature in thrombosis and inflammation. J Thromb Haemost. 2011; 9 (suppl 1): 168–173. [DOI] [PubMed] [Google Scholar]
- 50. Gonda Y,, Hirata S,, Saitoh K,, et al. Antithrombotic effect of recombinant human soluble thrombomodulin on endotoxin-induced disseminated intravascular coagulation in rats. Thromb Res. 1993; 71: 325–335. [DOI] [PubMed] [Google Scholar]
- 51. Taoka Y,, Okajima K,, Uchiba M,, Johno M. Neuroprotection by recombinant thrombomodulin. Thromb Haemost. 2000; 83: 462–468. [PubMed] [Google Scholar]
- 52. Kaneko H,, Joubara N,, Yoshino M,, et al. Protective effect of human urinary thrombomodulin on ischemia-reperfusion injury in the canine liver. Eur Surg Res. 2000; 32: 87–93. [DOI] [PubMed] [Google Scholar]
- 53. Ozaki T,, Anas C,, Maruyama S,, et al. Intrarenal administration of recombinant human soluble thrombomodulin ameliorates ischaemic acute renal failure. Nephrol Dial Transplant. 2008; 23: 110–119. [DOI] [PubMed] [Google Scholar]
- 54. Sharfuddin AA,, Sandoval RM,, Berg DT,, et al. Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol. 2009; 20: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eguchi Y,, Gando S,, Ishikura H,, et al. Post-marketing surveillance data of thrombomodulin alfa: sub-analysis in patients with sepsis-induced disseminated intravascular coagulation. J Intensive Care. 2014; 2: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]