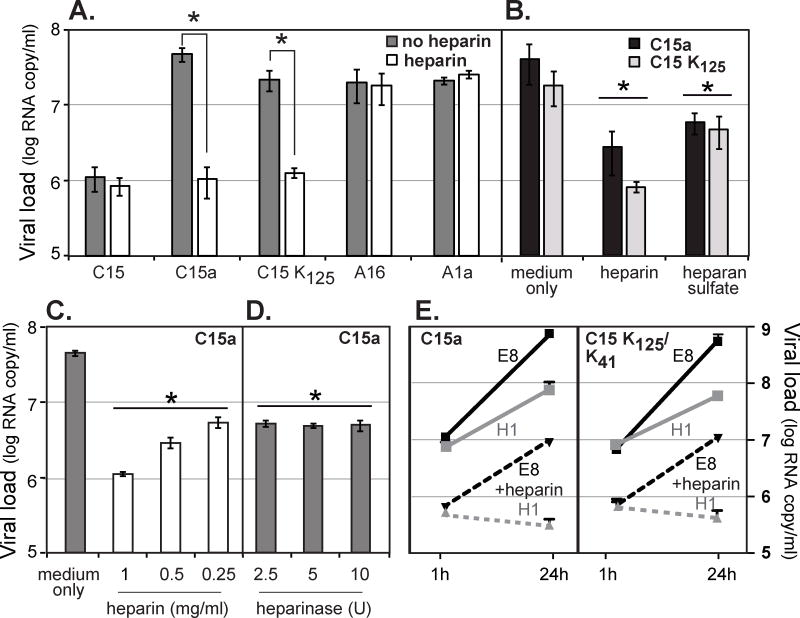
Figure 4.
Heparin and heparan sulfate effects. (A) Virus samples (108 vRNA copies) were incubated with heparin (2 mg/ml) or medium alone (no heparin) for 30 min at 34°C, before inoculation of HeLa-E8 cells. After an attachment period (30 min at room temp, 30 min at 34°C) the cells were washed (3× PBS), harvested and tested by RT-qPCR for the attached virus RNA signals (means ± s.d., n ≥ 3). (*) indicates p < 0.05 significance (t-test). (B) C15a and C15 K125 (108 vRNA copies) were incubated with heparin or heparan sulfate (1 mg/ml) as in A, before HeLa-E8 infections and virus binding assays. (C) The C15a experiment in A was repeated using different doses of heparin in the preincubation. (D) HeLa-E8 cells (12 well-plates) were treated (37°C, 2 h) with the indicated dose (U) of heparinase I before inoculation with C15a virus (108 vRNA copies) and tested for virus binding as in A. (E) C15a and recombinant C15 K125/K41 viruses (108 vRNA copies) were incubated with heparin (1 mg/ml, 30 min, 34°C) before inoculation of HeLa-E8 or HeLa-H1 cells. After an attachment period (30 min at room temp, 30 min at 34°C), the cells were washed with PBS (3×). Cells were harvested and assayed for the input virus (1 h p.i.) and progeny virus (24 h p.i.) RNA signals by RT-qPCR (means ± s.d., n = 3). In panels B, C, and D, (*) indicates p < 0.05 significance (t-test) vs. medium control sample.