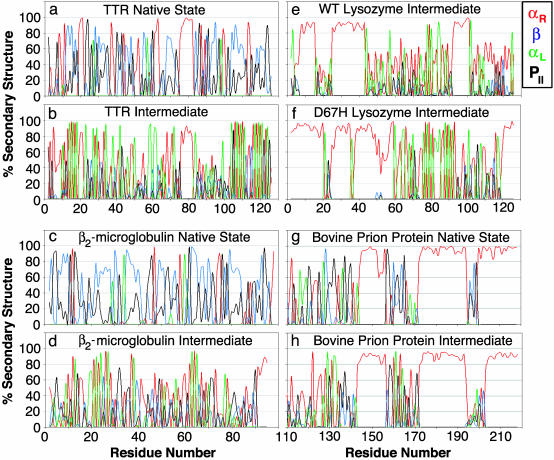
Fig. 2.
Average local secondary structure by residue. (a) Average over the first nanoseconds of the native simulation of TTR at 310 K. (b) Average over the α-sheet unfolding intermediate of TTR. (c) β2m at 310 K. (d) α-Sheet intermediate of β2m. (e) α-Sheet intermediate of WT lysozyme. (f) α-Sheet intermediate of D67H lysozyme. (g) Bovine PrP at 298 K. (h) α-Sheet intermediate of bovine PrP. A residue was classified in a particular conformation if its (φ,ψ) angles were within ± 30° of the average values that follow: αR = (45°, 92°); αL = (–87°, –49°); β-structure (both parallel and antiparallel) = (–165° ≤ φ ≤ –83°) and (89° ≤ ψ ≤ 169°); and PII = (–79°, 149°). Using these definitions there is some overlap between β and PII.