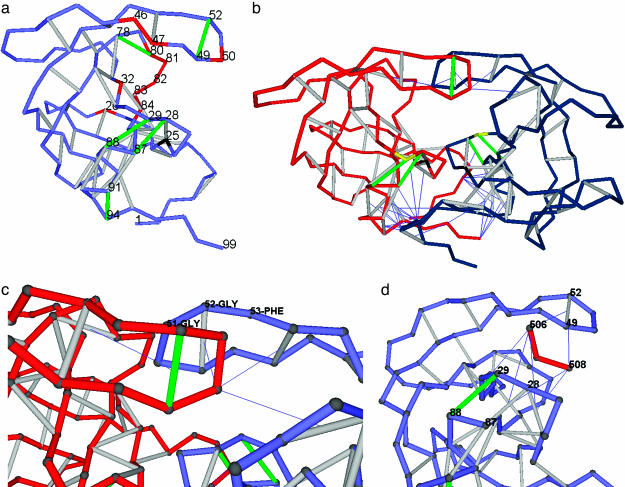
Fig. 1.
Distribution of dehydrons in HIV-1 protease monomer and dimer, flap asymmetry in the wrapping, and the inhibitor as a wrapper of packing defects. (a) Distribution of dehydrons in a monomeric HIV-1 protease unit. The backbone is represented as a light blue chain made up of virtual bonds joining α-carbons. Well wrapped backbone hydrogen bonds (see Methods) are indicated as gray segments joining α-carbons, and dehydrons are marked in green. The catalytically active D25 is shown in black, and the most significant residues undergoing site mutation associated with drug resistance or substrate specificity are shown in red. The dehydrons, together with their functional roles, are as follows: (G49, G52), flap flexibility, dimerization inducer; (G78-T80), induces dehydration of catalytic core; (A28, R87), stickiness of substrate-harnessing region and dimerization inducer; (D29, N88), stickiness of substrate-harnessing region; and (T91, G94), dimerization inducer. (b) Homodimeric HIV-1 protease (PDB entry 1A30) adopting the same virtual-bond backbone representation as in a, except that one monomer is shown in red and the other is shown in blue. Only intermolecular wrapping is shown. Thus, the line joining the α-carbon of a residue in a monomer and the center of a backbone hydrogen bond on the other monomer indicates penetration upon dimerization of at least one nonpolar group of the residue side chain into the desolvation domain of the hydrogen bond. Some dehydrons become well wrapped hydrogen bonds upon dimerization. The remaining dehydrons dictate the pathway of the peptide-chain substrate through the HIV-1 protease. The substrate-anchoring residue D29 is marked in yellow, and the catalytic D25 is marked in black, as above. (c) Detail revealing the broken symmetry upon induced fit in HIV-1 dimerization. The flap angle defined by α-carbons at positions G51, G52, and F53 is distorted in one of the monomers to better wrap the flap dehydron of the other intermolecularly. (d) The tripeptide inhibitor EDL (red, residues 506–508) wrapping the dehydrons (G49, G52), (A28, R87), and (D29, N88) in one monomer of HIV-1 protease within the dimer (PDB entry 1A30). Only (D29, N88) remains a dehydron (green) even upon further wrapping provided by the inhibitor.