Fig. 1. Models of Cofilin and GDI-1 function in invadopodial membrane trafficking in the C. elegans anchor cell.
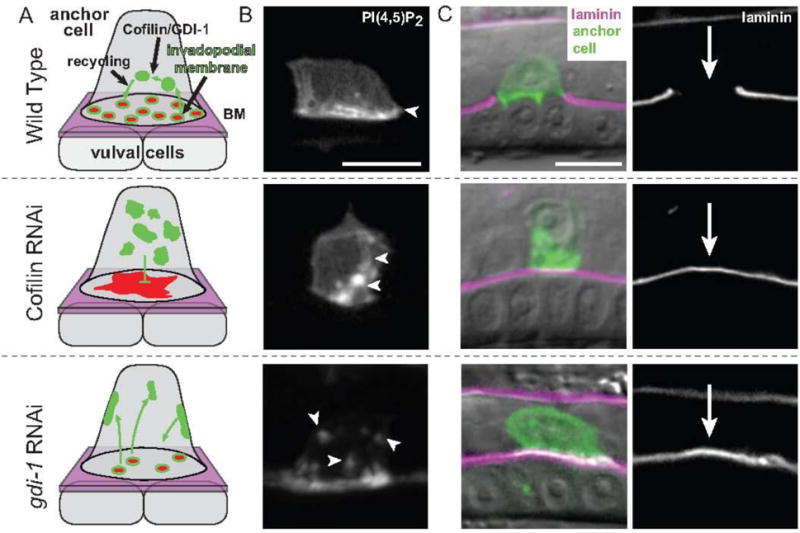
(A) Schematic images of invadopodial membrane trafficking within the anchor cell during anchor cell invasion into the vulval cells. F-actin and actin regulators (red) are coupled with invadopodial membrane trafficking (green) to form invadopodia at the anchor cell-basement membrane (BM, shown in magenta) interface prior to invasion. Both cofilin (encoded by the C. elegans unc-60a gene) and GDI-1 regulate trafficking of the invadopodial membrane through the endolysosome system. RNAi-mediated knockdown of cofilin results in a static, non-trafficked invadopodial membrane that is localized internally, an accumulation of stagnant F-actin along the invasive cell membrane, and an absence of invadopodia formation. Loss of GDI-1 results in mis-targeting of invadopodial membrane to all cell membranes and a reduction in the number of invadopodia. (B) A grayscale image of the invadopodial membrane observed with mCherry::PLCδPH, which binds to the invadopodial membrane component PI(4,5)P2 (highlighted by white arrows). In wild-type animals, the invadopodial membrane is localized along the invasive cell membrane. Loss of cofilin (through cofilin RNAi) results in a static, non-trafficked internal membrane and reduction of GDI-1 protein (through gdi-1 RNAi) causes the invadopodial membrane to be trafficked to all cell membranes. (C) Left, RNAi-mediated loss of the cofilin or GDI-1 results in invasion defects as seen by an inability of the anchor cell (green—visualized with either the invadopodial membrane probe mCherry::PLCδPH or the F-actin probe mCherry::MoeABD) to breach the basement membrane (magenta; visualized with laminin::GFP) overlaid on DIC. Right, a grayscale image of laminin::GFP. Arrow shows position of basement membrane breach in wild-type and a lack of breach after loss of cofilin or GDI-1. Bars, 5 μm.
