Abstract
To evaluate lung function responses to short-term indoor PM1 and PM2.5 concentrations, we conducted a panel study of healthy schoolchildren aged 13–14 years. The following lung function parameters FVC, FEV1, PEF, and mid expiratory flows MEF25, MEF50, and MEF75 were measured in 141 schoolchildren of the secondary school in Wroclaw, Poland in years 2009–2010. On days when spirometry tests were conducted, simultaneously, PM1 and PM2.5 samples were collected inside the school premises. Information about differentiating factors for children including smoking parents, sex, living close to busy streets, dust, mold, and pollen allergies were collected by means of questionnaires. To account for repeated measurements, the method of generalized estimating equations (GEE) was used. The GEE models for the entire group of children revealed the adverse effects (p < 0.05) of PM1 and PM2.5. Small differences in effects estimates per interquartile range (IQR) of PM1 and PM2.5 on MEF25 (5.1 and 4.8 %), MEF50 (3.7 and 3.9 %), MEF75 (3.5 and 3.6 %) and FEV1 (1.3 and 1.0 %) imply that PM1 was likely the component of PM2.5 that might have a principal health effect on these lung function parameters. However, the reduction of FVC and PEF per IQR for PM2.5 (2.1 and 5.2 %, respectively) was higher than for PM1 (1.0 and 4.4 %, respectively). Adjustment for potential confounders did not change the unadjusted analysis.
Keywords: Indoor PM pollution, School, Children, Panel study, Lung function
Introduction
Atmospheric particulate matter (PM) is the main component of air pollution in urban areas and has a significant impact on human health. The principal health effects include premature mortality, respiratory and cardiovascular diseases, and changes in lung function (WHO, 2005, WHO WHO, 2005a, WHO 2007). Children are more vulnerable to air pollution, because of greater ventilation rate per body weight and pulmonary surface area as compared to adults (Ginsberg et al. 2005; Bateson and Schwartz 2008). Deep breathing pulls air pollutants faster and further into the lungs—bypassing initial areas of deposition. The study by Ginsberg et al. (2005) has reported that the pulmonary region of the lung has slower clearance, thus particles remain there longer, meaning that the particle dose can be two- to fourfold higher among young children as compared to adults (Ginsberg et al. 2005).
The effect of air pollutants on lung function depends on the type of pollutant and its ambient concentration, duration of exposure and the total lung ventilation period of exposed individuals (D’Amato et al. 2010). Inhalable particles that can reach the lower airways are classified into three size fractions: PM10 (diameter ≤ 10 μm), PM2.5 (diameter ≤ 2.5 μm) and PM1 (diameter ≤ 1 μm). Particles included in PM10 but larger than 8 μm mostly remain in pharynx, larynx and trachea. Approximately 20 % of 10-μm particles penetrate through the extrathoracic airways and into the lower respiratory tract (Brown et al. 2013). Particles of 5–10 μm size reach upper parts of the bronchi, where they are removed in the process of mucociliary clearance, provided that the mucosa and cilia are intact (D’Amato et al. 2010). Unremoved particles affect the bronchial epithelium and muscularis mucosae of all parts of the bronchi, hence their importance should be considered with particular care. Although, the standard classification into PM10, PM2.5, and PM1 is broadly accepted, the complexity of the problem with classification of particles is still under discussion since the deposition of particle in lungs depends on various factors including sex, age, breathing habits, and activities (Brown et al. 2013).
Recent study suggests that 50 % of particles less than 4 μm in diameter penetrate into the lower respiratory tract in children (Brown et al. 2013). Other studies proved that particles with diameters equal or smaller than 2.5 μm (PM2.5) reach the alveoli and up to 50 % of them may remain in the lung tissue (Valavanidis et al. 2008). Fine PM can penetrate deep into the airways and induce alveolar inflammation, which is responsible for release of mediators favoring acute episodes of respiratory diseases (Schwartz 1992). Due to deep deposition they are removed very slowly, increasing the chances of causing cell damage (See and Balasubramanian 2008). Ultrafine particles are usually removed during the exhalation.
The association between exposure to ambient PM and reduced lung function parameters has been reported in many studies (Jedrychowski et al. 1999; Horak et al. 2002; Roy et al. 2012, Badyda et al. 2015). In recent decades, numerous panel studies of the influence of PM pollution on children’s lung function and respiratory symptoms were conducted and showed greater adverse effects of PM2.5 than PM10 on respiratory health outcomes in children (Ward and Ayres 2004; Li et al. 2012). The health effects of PM1 exposure were examined in only a few studies (Yu et al. 2000; Mar et al. 2004, Moshammer et al. 2006). Yu et al. (2000) found that the same-day PM1 concentrations were associated with asthma symptoms in children, Mar et al. (2004) identified a strong association between cough and both PM1 and PM2.5. In the studies of Moshammer et al. (2006), the changes per IQR were in the magnitude of 1 % for PM1.
The aim of this study was to assess the short-time effects of indoor dust particles with different aerodynamic diameters (PM1, PM2.5) on lung function data in healthy school children. While two indicators were considered, the analysis addressed whether a particular PM indicator better fitted to the data. Thus, the important issue was to identify the PM component with an acute effect.
Material and methods
Study population
The study was performed in the secondary school located in Wroclaw, a city in south-western Poland with population of approximately 700,000, near busy intersections (ca. 150 m). The school was chosen as the measurement site because it was located in a heavily populated residential area and the majority of the students resided in the area. Additionally, the school had sufficiently large student population. We reasoned that the children attending the school located in city center were likely to be exposed to indoor pollutants that infiltrate from outdoors and potentially may have negative impact on their health.
Similarly to other buildings in the area, the school was erected based on construction standards from the beginning of the last century, including no provisions for air conditioning system. Inside the building there is a huge patio (30 m long, 15 m wide, and 10 m high) covered with a glass roof. Corridors and classroom’s doors are placed along the perimeter of the patio on each floor. The classrooms are naturally ventilated via the doors, which are opened to the corridors during breaks. Thus, the air contained within the patio may be considered to be representative of the classroom condition.
The parents of the group of examined children (13–14 years old) had to provide their written informed consent for the participation of their child in the study and to fill out a questionnaire containing questions on respiratory and allergic diseases and home environment. Children with diagnosed bronchial asthma or other chronic respiratory diseases were excluded from the study. At the beginning, the study included 179 schoolchildren between 13 and 14 years old. We planned to test each healthy child ten times during the school year (2009/2010); however, as a result of school absences due to various reasons or symptoms of a common cold present on testing days, not all children were examined the intended number of times. In the end, our data base included 141 children with complete pulmonary function data from seven days during the school year 2009–2010.
Bioethics Committee of Wroclaw Medical University approval was obtained to conduct research in schoolchildren.
Lung function measurements
The children underwent lung function tests with the use of Blue Spiro portable spirometer (Micro 500; Medisoft Group). FVC, FEV1, PEF, and mid expiratory flows (MEF25, MEF50, MEF75) were obtained following the protocol of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) (Miller et al. 2005 ). All lung function data were expressed as percentage of the predicted normal values according to the ECCS/ERS reference equation.
Specifically, FVC, FEV1, PEF, and mid expiratory flows (MEF25%, MEF50%, and MEF75%) were measured in sitting position after at least 15 min of rest, while wearing a nose clip. A spirometry test was performed by trained professional medical staff and held in accordance with ATS/ERS procedures, except for the minimum exhalation time of 6 s, which is not feasible for most of the children (Arets et al. 2001). The results of spirometry were acceptable when FET was at least 3 s.
For each child, the aim was to get at least three acceptable maneuvers; however a maximum of eight attempts were allowed. Body weight and height were measured during the medical examination using calibrated measuring equipment.
The tests were performed for all participating children at the same time of day, between 9:00 and 14.00, once a month from December 2009 to October, 2010 (no measurements in July and August due to school holidays).
Short-term air pollution exposure assessment
The spirometry measurements were carried out in the hall of the building, which forms a part of the patio. The 8 h average of PM1 and PM2.5 (08:00–16:00) was considered in evaluation of lung function responses. These values included the lower concentrations during the lessons and the highest concentrations during the breaks. We assumed that short-term respiratory effects could be observed at the time of elevated levels of PM. Thus, the teaching hours average should represent the period of higher concentrations compared to the other times of the day. However, to characterize the overall indoor air pollution, PM1 and PM2.5 concentration measurements were performed on daily basis as 8 h means (08:00–16:00) and 16 h means (16:00–08:00) during the weekdays (from Monday to Friday) when lung functions were tested on the same days. Two Harvard cascade impactors (MS&T Area Samplers, AirDiagnostics and Engineering, Inc., Harrison, ME, USA) were used simultaneously. The pumps (Air Diagnostics and Engineering, model SP-280E) were set at airflow of 23 dm3/min for PM1 and 10 dm3/min for PM2.5. The volume of pumped air was controlled by Actaris-type flow meter. The particles were collected by 37 mm diameter Teflon membrane filters (PALLFLEX, TK15-G3 M). All filters were pre- and post-conditioned in a clean room with environmentally controlled temperature and humidity prior to weighing. Weighing was carried out with an electronic microbalance (Santorius M5P 000 V001) with ±1 μg sensitivity and in the 500 mg range. The analysis was carried out in accordance with European PM Marking Standard (NBN-EN-12,341). Before weighing, filters were conditioned for 48 h at 20 °C.
Covariates
Data on inter-child differences such as smoking parents at the child’s home (SMOKERS), sex (SEX), living on the main city street (STREET), dust allergy (DUST), pollen allergy (POLLEN), molds allergy (MOLD), dampness in the child’s home (DAMP), and traffic nuisance due to the passage of trucks (TRAFFIC) were collected by means of questionnaires. The environmental conditions such as temperature or humidity were nearly constant inside the school during the study period; therefore, these variables were not considered as potential covariates. Air temperature, humidity, and air velocity were checked using Mini HydroThermo-Anemometr (Extech Instruments) four times a day throughout the sampling periods. The indoor temperature varied slightly from 18 to 21 °C, regardless of the season; relative humidity ranged between 31 and 54 %; air velocity was below the detection limit (0.5 m/s).
Statistical analysis
We used lung function parameters (outcome variables) as dependent variables to analyze their response to PM pollution. An outcome variable is a percent of the value rather than an absolute value. Thus, lung function values were log-transformed because of expected multiplicative effects (Moshammer et al. 2006). To account for repeated measurements, the method of generalized estimating equations (GEE – single pollutant model) was used with an autoregressive working correlation matrix. The same group of schoolchildren (141) was tested seven times in different exposures to indoor PM. GEE is one of the most frequently used statistical method in such panel studies (Li et al. 2012). Data entry and analyses were done with R package geepack Version 3.1 (http://www.r-project.org/). Statistical significance was determined using a p-value of 0.05.
All effect estimates from GEE models (β coefficients, 95 % CI) were transformed into percent change of lung function parameters measured per interquartile range (25–75 %; IQR) of the respective PM pollutant using the formula (eβ*IQR – 1)×100.
A univariate GEE model was also run to provide information for inter-child differences and to examine the impact of each covariate on each lung function parameter. Covariates were defined as dichotomous variables.
Finally, we performed a multivariate approach of GEE for each PM pollutant and those covariates that were significantly (p < 0.05) associated with at least one of the outcome variables in univariate models.
Results
Characteristic of the study population
Characteristics of the study population and the distribution of lung function parameters are presented in Table 1 and in Fig. 1.
Table 1.
Population characteristics (n = 141)
| Variable | N | Percent (%) |
|---|---|---|
| Female SEX | 91 | 64.5 |
| Smoking at child’s home SMOKERS | 51 | 36.2 |
| Dampness at child’s home DAMPNESS | 5 | 3.5 |
| Living on the main city street STREET | 57 | 40.4 |
| Pollen allergy POLLEN | 35 | 24.8 |
| Dust allergy DUST | 24 | 17.0 |
| Mold allergy MOLD | 3 | 2.1 |
| Passage of trucks nuisance TRAFFIC | 54 | 38.2 |
Fig. 1.
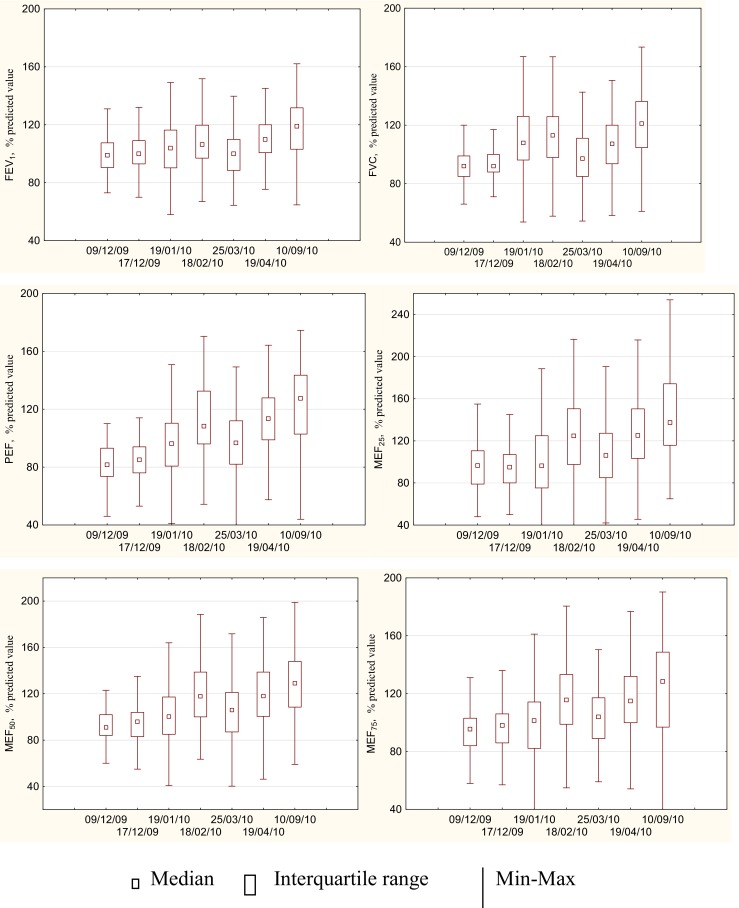
Descriptive statistics of lung function parameters among schoolchildren (n = 141) in following testing days
Using the dependent t test (the same group of children was tested repeatedly over time), we compared the means of lung function parameters collected on different testing days to detect whether there were any statistically significant differences between these means. Before doing this, we made sure that our data met assumptions of the test. With p-values greater than 0.05 (p > 0.05), we could conclude that there were no significant differences between the means of PEF and MEF in December and January, and March, as well as in February and April, and September which essentially states that there were significant differences in means between December and February, and April, and September. There were significant differences in means for FEV1 between December and only April, and September; for FVC between December and all remaining testing days.
Using the univariate GEE approach, we estimated associations for FEV1, FVC, PEF, MEF25, MEF50, MEF75 and potentially cofounders: SEX, SMOKING, DAMPNESS, STREET, DUST, POLLEN, MOLD, and TRAFFIC (Table 2). Associations between all six lung function parameters and SMOKING, DAMPNESS, POLLEN, and MOLD were not statistically significant. Negative and significant associations were observed for SEX (boys). Associations showed the discrepancy for STREET, DUST, and TRAFFIC. There were significant (p < 0.05) negative associations between PEV1 and PEF and children feeling traffic nuisance, TRAFFIC, and between FEV and MEF50 and children living near heavy traffic roads, STREET, and between MEF50 and children allergic to house dust, DUST. Nevertheless, all these covariates, e.g., SEX, STREET, DUST, and TRAFFIC were considered in a multivariate approach of GEE for each PM pollutant.
Table 2.
Effects estimates (β, 95 % CI) and probability level (p) for the associations of logFEV1, logFVC, logPEF, logMEF25, logMEF50, logMEF75 and potentially confounders (italics with p < 0.05)
| FEV1 | FVC | PEF | MEF25 | MEF50 | MEF75 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
β
(95%CI) |
P |
β
(95%CI) |
p |
β
(95%CI) |
P |
β
(95%CI) |
P |
β
(95%CI) |
p |
β
(95%CI) |
p | |
| SEX (boys) |
-0.027
(−0,045; -0.009) |
<0.05 |
-0.039
(−0.060, - 0.019 |
<0.05 |
-0.028
(−0.048, -0.007) |
<0.05 |
-0.058
(−0.087, -0.029) |
<0.05 |
-0.046
(−0.070, -0.022) |
<0.05 |
-0.057
(−0.078, -0.035) |
<0.05 |
| SMOKING | -0.012 (−0.030, 0.007) |
0.22 | -0.010 (−0.031, 0.011) |
0.35 | -0.007 (−0.027, 0.015) |
0.54 | -0.011 (−0.041, 0.015) |
0.48 | -0.012 (−0.037, 0.013) |
0.95 | -0.007 (−0.030, 0.016) |
0.56 |
| DAMPNESS | 0.013 (−0.027, 0.053) |
0.53 | 0.015 (−0.032, 0.061) |
0.53 | 0.030 (0.000, 0.060) |
0.06 | 0.013 (−0.046, 0.073) |
0.67 | 0.020 (−0.027, 0.067) |
0.41 | 0.012 (−0.027, 0.052) |
0.55 |
| STREET | -0.014 (−0.031, 0.003) |
0.10 | -0.014 (−.0033, 0.006) |
0.16 |
-0.02
(−0.040, -0.001) |
0.04 | -0.014 (−0.042, 0.015) |
0.34 |
-0.030
-0.053, -0.006 |
0.02 | -0.022 (−0.045. 0.000) |
0.05 |
| DUST | 0.010 (−0.012, 0.033) |
0.37 | 0.008 (−0.018, 0.035) |
0.52 | 0.016 (−0.009, 0.041) |
0.20 | 0.012 (−0.026. 0.049) |
0.55 |
0.037
(−0.009, 0.065) |
0.01 | 0.027 (−0.001, 0.056) |
0.06 |
| POLLEN | -0.002 (−0.021, 0.016) |
0.80 | -0.006 (−0.028, 0.015) |
0.56 | 0.003 (−0.019, 0.025) |
0.78 | 0.011 (−0.020, 0.043) |
0.49 | 0.004 (−0.020, 0.028) |
0.73 | 0.005 (−0.021, 0.031) |
0.72 |
| MOLD | -0.002 (−0.030, 0.026) |
0.89 | -0.002 (−0.050, 0.046) |
0.93 | -0.017 (−0.062, 0.041) |
0.68 | -0.029 (−0.083, 0.025) |
0.30 | -0.003 (−0.043, 0.038) |
0.89 | 0.000 (−0.058, 0.057) |
0.99 |
| TRAFFIC |
-0.028
(−0.055, -0.001) |
0.04 | -0.014 (−0.045, 0.018) |
0.39 |
-0.034
(−0.066, -0.002) |
0.04 | 0.000 (−0.033, 0.034) |
0.98 | 0.000 (−0.034, 0.033) |
0.98 | -0.030 (−0.065, 0.006) |
0.11 |
2.2. Indoor PM1, and PM2.5 concentrations
The means, standard deviations and IQRs of the concentrations for the spirometry days (test days) are shown in Table 3. How did these values relate to the average concentrations in school? Thus, 8-h mean levels at the testing days (Table 3) were comparable to daily mean PM for the whole sampling period, covering the weeks, when the children were tested in a single day (Fig. 2). It follows that the short-term effects of PM on lung function were investigated across a typical range of school environmental conditions (and hence different exposure of PM1 and PM2.5).
Table 3.
Means, standard deviations and interquartile ranges (IQR) of short-term (8-h average) exposures to PM inside the school building during the spirometry days (n = 7)
| PM fraction | Mean(standard deviation), μg/m3 | Interquartile range (IQR), μg/m3 |
|---|---|---|
| PM1 | 22 (8) | 9 |
| PM2.5 | 67 (43) | 41 |
Fig. 2.
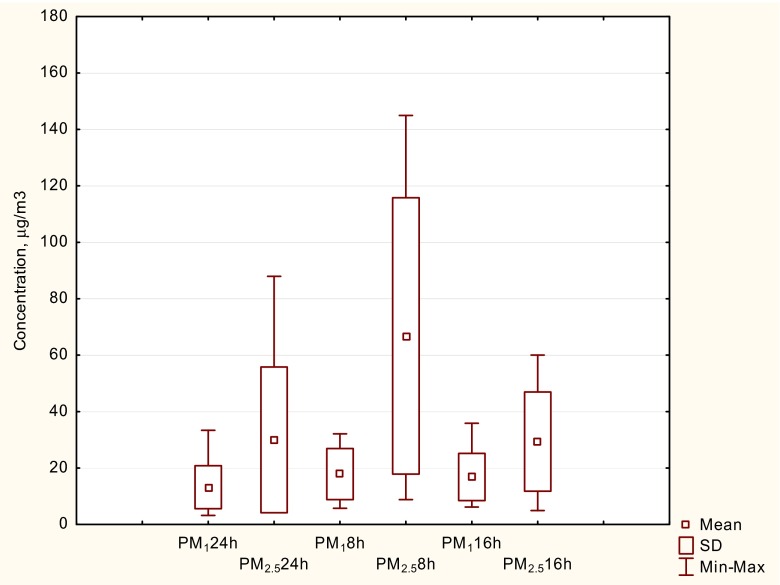
A box-and-whisker plot of 24 h (PM2 4 h ), 8 h (PM8 h) and 16 h (PM1 6 h) data set (the whole sampling period, covering these weeks, when the children were tested in a single day)
Figure 2 shows the box plots representing the distribution of 24 h (PM2 4 h ), 8-h (PM8 h)n and 16-h (PM1 6 h) mean concentrations of PM1 and PM2.5 inside the school hall during the whole sampling period. Nightly concentrations were the indicators for the strength of indoor sources during the teaching hours. Thus, the findings indicate that indoor dust sources caused of elevated PM2.5 concentrations during daytime as compared to nighttime (ca. three times higher). PM1 concentrations reached comparable levels for both sampling periods. Additionally, there were significant but not strong correlation between PM1 and PM2.5 indoor concentrations (R 2 = 0,34; p = 0.045), implying that it will be possible to identify the PM component that may have the adverse effect. The small R 2 value means that there were likely different sources of origin for PM2.5 and PM1. Details of the contributions of outdoor and indoor sources to the indoor concentrations of PM1 and PM2.5 have been described by Zwoździak et al. 2013, 2014.
2.3. Lung function responses to PM pollution
The associations between indoor 8 h averages of PM1 and PM2.5 concentrations and lung function parameters, FEV1, FVC, PEF, MEF25, MEF50, and MEF75, within the schoolchildren population estimated by the single pollutant GEE model is shown in Table 4. In all analyses, PM1 and PM2.5 were significant predictors (p < 0.05); the negative associations suggested decreases in lung function with increasing exposure. In general, the comparison of effect estimates suggests a greater effect for PM1 compared to PM2.5. Overall effect estimates were small only for FEV1, in the magnitude of 1 % per IQR. The strongest changes were seen for PEF and in maximal expiratory flow in small airways (MEF’s), in the magnitude of 4–5 % per IQR. As PM1 was a part of PM2.5 (from 50 to 82 %, on average 70 % of PM2.5 inside school during testing days) and very small differences in effects estimates for PM1 and PM2.5 observed (except for FVC and PEF) implied that mainly PM1 may have the adverse effect, while exposure to dust PM2.5 did not substantially reduce lung function as compared to PM1, particularly in maximal expiratory flow in small airways (MEF).
Table 4.
Percent change (%) and 95 % CI in lung function parameters per interquartile change range (IQR) of the respective PM pollutant (results from single pollutant GEE models, p < 0.05)
| PM fraction | FEV1 | FVC | PEF | MEF25% | MEF50% | MEF75% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %/ IQR |
95%CI | %/ IQR |
95%CI | %/ IQR |
95%CI | %/ IQR |
95%CI | %/ IQR |
95%CI | %/ IQR |
95%CI | |
| PM1 | −1.3 | −1.8, −0.8 | −1.0 | −1.5, −0.5 | −4.4 | −5.0, −3.7 | −5.1 | −5.8, −4.5 | −3.7 | −4.5, −3.0 | −3.5 | −4.2, −2.8 |
| PM2.5 | −1.0 | −1.4, −0.6 | −2.1 | −2.6, −1.6 | −5.2 | −5.7, −4.7 | −4.8 | −5.4, −4.2 | −3.9 | −4.5, −3.2 | −3.6 | −4.2, −3.0 |
Final results from the multivariate GEE model are presented in Appendix (Table 5) because the estimated percent change of a given outcome variable per IQR increase in the exposure to each air pollutant, controlling for sex (SEX), living close to busy streets (STREET), dust allergy (DUST) and traffic nuisance (TRAFFIC) remained negligible changed compared to the single pollutant analysis. It indicates that adjustment for potential confounders did not change the unadjusted analysis and small changes of lung function parameters after exposure to PM pollution were observed, despite personal home environment and allergies. We adjusted statistical parameters for known potential confounders (Table 2), but the possibility of confounding by other factors still exists (including temperature, season, and other air pollutants).
Table 5.
Adjusted for SEX, DUST, STREET, and TRAFFIC associations between 8-h average PM levels and lung function parameters (results from multivariate GEE models, p < 0.05)
| PM fraction | FEV1 | FVC | PEF | MEF25% | MEF50% | MEF75% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %/ IQR |
95%CI | %/ IQR |
95%CI | %/ IQR |
95%CI | %/ IQR |
95%CI | %/ IQR |
95%CI | %/ IQR |
95%CI | |
| PM1 | −1.3 | −1.7, −0.8 | −1.0 | −1.6, −0.5 | −4.4 | −5.0, −3.7 | −5.1 | −5.9, −4.5 | −3.8 | −4.5, −3.0 | −3.6 | −4.3, −2.9 |
| PM2.5 | −1.0 | −1.4, −0.6 | −2.1 | −2.6, −1.6 | −5.2 | −5.7, −4.7 | −4.7 | −5.3, −4.1 | −3.9 | −4.5, −3.2 | −3.5 | −4.1, −2.9 |
Abbreviations:
ECCS - European Community of Coal and Steel
Discussion
The main interest of the investigation was whether short-term PM (PM1, PM2.5) concentrations had an impact on lung function parameters of healthy schoolchildren. The measured daily 8-h mean PM2.5 concentrations inside the school building were nearly two times higher than the calculated 24-h means. Air quality standards are based on outdoor PM2.5 concentrations. There is no legal limit value for outdoor PM1 and indoor PM2.5 and PM1 yet. The mean 24-h indoor concentration of PM2.5 was 37 μg/m3 in the school under investigation. This is consistent with several other studies that have shown that PM2.5 concentrations in classrooms were high and exceeded the World Health Organization Air Quality Guidelines (WHO 2005a) for the ambient air (Ekmekcioglu and Keskin 2007; Diapouli et al. 2008; Oeder et al. 2012). The short-term WHO AQG for PM2.5 is 25 μg/m3 (24-h means). Notably, children usually spend approximately 6–8 h in school and are therefore exposed to high levels of PM.
The results presented in Table 4 suggest that high indoor PM levels can lead to reduced values of lung function parameters: FEV1, FVC, PEF, MEF25, MEF50, and MEF75. The associations were statistically significant (p < 0.05) for PM fractions studied, however differences in effect estimates were observed both for the PM size and measured outcome variables. The changes per IQR were in the range from 1 to 2 % only for FEV1 and FVC. The strongest changes per IQR, from 3.5 to 5.2 %, were observed for PEF and MEF25, MEF50 and MEF75. As compared to the study carried out in Linz, Austria (Moshammer et al. 2006), the changes in spirometric parameters of schoolchildren were higher in our study. In Austria, 163 healthy children aged 7–10 were tested once a month throughout 1 year. The spirometric parameters were tested in relation to 8 h mean concentration of PM1 recorded at a nearby monitoring station. Using the GEE method, the lung function deficits per IQR were in the magnitude of 1 % for PM1. The interquartile range was 10.9 μg/m3, in our study 9.0 μg/m3, respectively but the 8 h mean was 12.3 μg/m3, i.e., lower than in our study – 22.0 μg/m3.
The study of Chan et al. (2015) has provided evidence that exposure to current levels of ambient PM2.5 in Taiwan was associated with reductions in children’s lung function (FEV1, FVC). They performed spirometry in 1494 healthy children aged 6–15 years from 44 schools. Air pollution data were obtained from air monitoring stations within 1 km of the schools. Authors concluded that sub-chronic exposure to ambient PM2.5 (mean concentration: 38.6 μg/m3) reduces lung capacity in children aged 6–15 years and may induce additional airway obstructive patterns of lung function in children aged 6–10 years. In contrast, Epton et al. (2008) who studied the effect of ambient particulate air pollution on the respiratory function of male schoolchildren (93 students) in New Zealand concluded that only asthmatic children showed small effects of high PM10 levels on FEV1 and PEF. No significant effects were observed for healthy children. The majority of PM10 pollution was in the PM2.5 range and indoor pollution levels were similar to outdoor.
A review paper of panel studies in children did not provide a clear association of lung function with increasing PM pollution (Ward and Ayres 2004). Ward and Ayres 2004 concluded that a considerable diversity of results was observed. However, a small adverse effect of PM2.5 for PEF was shown. Healthy children appeared to be more affected by PM levels than those with diagnosed respiratory symptoms. This finding was in contrast to a literature review by Li et al. (2012). They concluded that more serious adverse effects of PM on lung function parameters were observed for asthmatic children.
In many panel studies, PEF and FEV1 were the most frequently measured parameters to assess the effects of PM2.5 or PM10 pollution on children’s lung function (Ward and Ayres 2004; Li et al. 2012; Jacobson et al. 2012; Chan et al. 2015). Despite the fact that the smallest fraction penetrates the deepest into the airways, the health effects of PM1 on lung function in children have been poorly investigated. Thus, the strength of our study was that two fractions of PM (PM1 and PM2.5) and six different spirometry parameters (FEV1, FVC, PEF, MEF25, MEF50, and MEF75) were measured simultaneously and their average associations within the schoolchildren population were estimated. We observed negative impact of both indoor PM1 and PM2.5 on lung function parameters of healthy schoolchildren. It should be emphasized that indoor PM1 accounted on average for 70 % of PM2.5 mass concentrations during testing days.
Overall, our finding of a decrease in lung function with increasing exposure to PM1 and PM2.5 is consistent not only with other short term studies, but also with long term studies such as cohort studies in Europe, ESCAPE study (Gehring et al. 2013), which have confirmed the adverse effect of PM2.5 pollution. For example, statistically significant decreases in PEF, 0.8 % per 5 μg/m3 increase in PM2.5 was found in a Norwegian population study (Oftedal et al. 2008).
Important limitations to our study must be noted. Firstly, the sample size was fairly small: individual level observations of lung function in 141 schoolchildren and PM pollution records were collected for 7 days, although, across varied exposure levels of PM1 and PM2.5. Thus, attempts at generalization should be done with caution. Secondly, the observed effects may be biased by the assumption of the exposure time and teaching hours. The 8-h mean PM concentration on the days of spirometry measurements, was assumed to be suitable for this kind of study. To assess a group level function: concentration- response (impact on children respiratory system as a whole, we did not consider this relationship for individual child), data are needed that characterize concentrations when children are at school. In short-term effect studies, the largest effects have been often reported for air pollution levels on the day when the lung function measurements were performed or on the days preceding the examination (Gehring et al. 2013). We realize that the best estimates require the use of monitoring devices that can be carried by pupils. This is the direction for our further studies. Further research is needed to examine the lag effects of PM pollution on children’s lung function and interactive effects between PM concentrations and indoor humidity.
Conclusions
Exposure to elevated PM concentrations causes a decrease in the lung function parameters in healthy schoolchildren resulting in poorer spirometry results. According to WHO (http://www.euro.who.int/) there is no evidence of a safe level of exposure or a threshold below which no adverse health effects of PM occur.
We observed the greater effect for PM1 compared to PM2.5 on the lung function parameters. PM1 fraction is likely to be the better indicator for acute effects than PM2.5 fraction indoor (at the school) and involves pronounced changes in schoolchildren lung function mainly in small airways. PM1 mass concentrations are not routinely monitored at most air pollution monitoring stations but may be more important for health effects than bigger size PM fractions.
The negative impact of indoor PM1 and PM2.5 pollution on healthy children lung function requires an effective indoor air quality management program, which is necessary to reduce children’s health risks to a minimum.
Acknowledgments
Special thanks to the school manager and teachers of Secondary School No. 13 in Wroclaw for their interest in this work and valuable support.
Appendix
Compliance with ethical standards
Grant support
This work was supported by the National Science Centre under the project no. N304 067,937 “Effects of air pollution on short-term pulmonary function changes in schoolchildren”.
Competing interest
The authors declare that they have no competing interests.
Human subjects research
This study was approved by the Bioethics Committee of Wroclaw Medical University (nr KB-619/2007, 03.01.2008), additionally the parents of each participant provided informed consent.
References
- Arets HGM, Brackel HJL, van der Ent CK. Forced expiratory manoeuvres in children: do they meet ATS and ERS criteria for spirometry? Eur Respir J. 2001;18:655–660. doi: 10.1183/09031936.01.00204301. [DOI] [PubMed] [Google Scholar]
- Badyda A, Dabrowiecki P, Czechowski P, Majewski G. Risk of bronchi obstruction among non-smokers – review of environmental factors affecting bronchoconstriction. Resp Physiol Neurobi. 2015;209:39–46. doi: 10.1016/j.resp.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health A. 2008;71:238–243. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- Brown JS, Gordon T, Price O, Asgharian B. Thoracic and respirable particle definitions for human health risk assessment. Part Fibre Toxicol. 2013;10(10):12. doi: 10.1186/1743-8977-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Chen BY, Cheng TJ, Leon Guo Y. Effects of particulate air pollution and ozone on lung function in non-asthmatic children. Environ Res. 2015;137:40–48. doi: 10.1016/j.envres.2014.11.021. [DOI] [PubMed] [Google Scholar]
- D’Amato G, Cecchi L, D’Amato M, Liccardi G. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J Investig Allergol Clin Immuno. 2010;l20:95–102. [PubMed] [Google Scholar]
- Diapouli E, Chaloulakou A, Mihalopoulos N, Spirellis N. Indoor and outdoor PM mass and number concentrations at schools in the Athens area. Environ Monit Assess. 2008;136:13–20. doi: 10.1007/s10661-007-9724-0. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu D, Keskin SS. Characterization of indoor air particulate matter in selected elementary schools in Istanbul, Turkey. Indoor Built Environ. 2007;16:169–176. doi: 10.1177/1420326X07076777. [DOI] [Google Scholar]
- Epton MJ, Dawson RD, Brooks WM, Kingham S, Aberkane T, Cavanagh J-A, et al. The effect of ambient air pollution on respiratory health of school children: a panel study. Environ Health. 2008;7 doi: 10.1186/1476-069X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, Gruzieva O, Agius RM, Beelen R, Custovic A, Cyrys J, et al. Air pollution exposure and lung function in children: the ESCAPE project. Environ Health Perspect. 2013;121:1357–1364. doi: 10.1289/ehp.1306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg GL, Foos BP, Firestone MP. Review and analysis of inhalation dosimetry methods for application to children’s risk assessment. J Toxicol Environ Health A. 2005;68:573–615. doi: 10.1080/15287390590921793. [DOI] [PubMed] [Google Scholar]
- Jacobson LSV, Hacon SS, Castro HA, Ignotti E, Artaxo P, Leon ACMP. Association between fine particulate matter and the peak expiratory flow of schoolchildren in the Brazilian subequatorial Amazon: a panel study. Environ Res. 2012;117:27–35. doi: 10.1016/j.envres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Flak E, Mróz E. The adverse effect of low levels of ambient air pollutants on lung function growth in preadolescent children. Environ Health Perspect. 1999;107:669–674. doi: 10.1289/ehp.99107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Williams G, Jalaludin B, Baker P. Panel studies of air pollution on children’s lung function and respiratory symptoms: a literature review. J Asthma. 2012;49:895–910. doi: 10.3109/02770903.2012.724129. [DOI] [PubMed] [Google Scholar]
- Mar TF, Larson TV, Stier RA, Claiborn C, Koenig JQ. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhal Toxicol. 2004;16:809–815. doi: 10.1080/08958370490506646. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi S, Coates A, et al. Standardisation of spirometry. Eur Respir. 2005;J26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Moshammer H, Hutter HP, Hauck H, Neuberger M. Low levels of air pollution changes of lung function in a panel of schoolchildren. Eur Respir. 2006;J27:1138–1143. doi: 10.1183/09031936.06.00089605. [DOI] [PubMed] [Google Scholar]
- Oeder S, Dietrich S, Weichenmeier I, Schober W, Pusch G, Jorres RA, et al. Toxicity and elemental composition of particulate matter from outdoor and indoor air of elementary schools in Munich, Germany. Indoor Air. 2012;22:148–158. doi: 10.1111/j.1600-0668.2011.00743.x. [DOI] [PubMed] [Google Scholar]
- Oftedal B, Brunekreef B, Nystad W, Madsen C, Walker SE, Nafstad P. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology. 2008;19:129–137. doi: 10.1097/EDE.0b013e31815c0827. [DOI] [PubMed] [Google Scholar]
- Roy A, Hu W, Wei F, Korn L, Chapman RS, Zhang JJ. Ambient particulate matter and lung funtion growth in Chinese children. Epidemiology. 2012;23:464–472. doi: 10.1097/EDE.0b013e31824cbd6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. Particulate air pollution and daily mortality: a synthesis. Public Health Rev. 1992;19:39–60. [PubMed] [Google Scholar]
- See S, Balasubramanian R. Chemical characteristics of fine particles emitted from different gas cooking methods. Atmos Environ. 2008;42:8852–8862. doi: 10.1016/j.atmosenv.2008.09.011. [DOI] [Google Scholar]
- Horak F, Jr, Studnicka M, Gartner C, Spengler JD, Tauber E, Urbanek R, et al. Particulate matter and lung function growth in children: a 3-yr follow-up study in Austrian schoolchildren. Eur Respir J. 2002;19:838–845. doi: 10.1183/09031936.02.00512001. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- Ward DJ, Ayres JG. Particulate pollution and panel studies in children; a systematic review. Occup Environ Med. 2004;61:e13. doi: 10.1136/oem.2003.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2005a) Health effects of transport-related air pollution. Copenhagen, WHO regional Office for Europe (http://www.euro.who.int/data/assets/pdf_file/0006/74715/E86650.pdf, accessed 29 June 2016)
- WHO (2005b) Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Global update 2005( http://www.euro.who.int/data/assets/pdf_file/0005/7838/E90038, accessed 29 June 2016)
- WHO (2007) Health revelance of particulate matter from various sources. Bonn, Germany, WHO Regional Office for Europe (http://www.euro.who.int/__data/assets/pdf_file/0007/78658/E90672.pdf, accessed 29 June 2016)
- Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in Seattle area children enrolled in the CAMP study. Environ Health Perspect. 2000;108:1209–1214. doi: 10.1289/ehp.001081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwoździak A, Sówka I, Krupińska B, Zwoździak J, Nych A. Infiltration or indoor sources as determinants of the elemental composition of particulate matter inside a school in Wrocław, Poland? Build Environ. 2013;66:173–180. doi: 10.1016/j.buildenv.2013.04.023. [DOI] [Google Scholar]
- Zwoździak A, Sówka I, Worobiec A, Zwoździak J, Nych A. The contribution of outdoor particulate matter (PM1, PM2.5, PM10) to school indoor environment. Indoor Built Environ. 2014 [Google Scholar]