We investigate the underlying neurophysiology of age-related auditory temporal processing deficits in normal-hearing listeners using two different types of noise: comprehensible and incomprehensible natural speech. Two neurophysiological techniques are used—magnetoencephalography and EEG—to investigate two different brain areas—cortex and midbrain—within each participant. Older adults' cortical and midbrain responses depend more critically on noise level and are more affected by the type of noise than younger adults' responses.
Keywords: aging, electrophysiology, midbrain, cortex, hearing
Abstract
The ability to understand speech is significantly degraded by aging, particularly in noisy environments. One way that older adults cope with this hearing difficulty is through the use of contextual cues. Several behavioral studies have shown that older adults are better at following a conversation when the target speech signal has high contextual content or when the background distractor is not meaningful. Specifically, older adults gain significant benefit in focusing on and understanding speech if the background is spoken by a talker in a language that is not comprehensible to them (i.e., a foreign language). To understand better the neural mechanisms underlying this benefit in older adults, we investigated aging effects on midbrain and cortical encoding of speech when in the presence of a single competing talker speaking in a language that is meaningful or meaningless to the listener (i.e., English vs. Dutch). Our results suggest that neural processing is strongly affected by the informational content of noise. Specifically, older listeners' cortical responses to the attended speech signal are less deteriorated when the competing speech signal is an incomprehensible language rather than when it is their native language. Conversely, temporal processing in the midbrain is affected by different backgrounds only during rapid changes in speech and only in younger listeners. Additionally, we found that cognitive decline is associated with an increase in cortical envelope tracking, suggesting an age-related over (or inefficient) use of cognitive resources that may explain their difficulty in processing speech targets while trying to ignore interfering noise.
NEW & NOTEWORTHY
We investigate the underlying neurophysiology of age-related auditory temporal processing deficits in normal-hearing listeners using two different types of noise: comprehensible and incomprehensible natural speech. Two neurophysiological techniques are used—magnetoencephalography and EEG—to investigate two different brain areas—cortex and midbrain—within each participant. Older adults' cortical and midbrain responses depend more critically on noise level and are more affected by the type of noise than younger adults' responses.
human ability to segregate speech in noisy environments significantly degrades with aging, even when hearing acuity is clinically normal (Burke and Shafto 2008; Getzmann et al. 2015). The results of several behavioral studies have suggested that older adults have problems processing auditory temporal information in a number of tasks, such as gap-in-noise detection and distorted speech (Fitzgibbons and Gordon-Salant 1996, 2001; Frisina and Frisina 1997; Gordon-Salant et al. 2006; He et al. 2008; Pichora-Fuller and Schneider 1991; Schneider and Hamstra 1999). Electrophysiological studies have shown that these behavioral problems reflect a change in latency and strength of the auditory midbrain- and cortical-evoked responses (Anderson et al. 2012; Clinard and Tremblay 2013; Lister et al. 2011; Parthasarathy and Bartlett 2011; Presacco et al. 2015). A number of animal studies have also suggested that these problems may stem from an age-related imbalance between the inhibitory and excitatory processes in the dorsal cochlear nucleus (Caspary et al. 2005; Schatteman et al. 2008; Wang et al. 2009), inferior colliculus (Caspary et al. 1995), spiral ganglion neurons (Tang et al. 2014), and auditory cortex (de Villers-Sidani et al. 2010; Hughes et al. 2010; Juarez-Salinas et al. 2010).
This difficulty may arise, in part, from different effects of noise on neural speech encoding in younger vs. older adults (Billings et al. 2015; Presacco et al. 2016). Specifically, neural synchronization in the midbrain and cortex is deteriorated by noise to a greater extent in older adults. The cortical response also revealed an age-related, abnormally high (over-representation) response in older adults in both quiet and noise conditions. These results suggest a disruption of the normal balance between excitatory and inhibitory processes and are consistent with several studies showing age-related auditory temporal processing deficits both in the midbrain (Anderson et al. 2012; Caspary et al. 1995, 2005, 2006; Parthasarathy and Bartlett 2011; Parthasarathy et al. 2010; Presacco et al. 2015; Walton et al. 1998) and in the cortex (Alain et al. 2014; Getzmann et al. 2016; Getzmann and Naatanen 2015; Lister et al. 2011; Ross et al. 2010; Soros et al. 2009) that would be exacerbated by the presence of hearing loss (Anderson et al. 2013; Henry et al. 2014; Humes and Christopherson 1991; Humes and Roberts 1990; Peelle et al. 2011).
Additionally, several studies have shown how the amplitude and the latency of the neural response are significantly affected by the presence and level of noise in the midbrain (Anderson et al. 2010; Burkard and Sims 2002) and cortex (Billings et al. 2013, 2015; Ding and Simon 2013). Specifically, amplitude decreases with noise, whereas the latency of the main peaks (e.g., wave V of the brain stem and P1, N1, and P2 in the cortex) of the evoked response becomes longer.
Despite this age-related neural decline, the ability of older adults to follow a conversation is not entirely compromised, as they seem to rely more heavily on the use of contextual cues than do younger adults and seem to be differently affected by the informational and energetic content of the background noise. Specifically, several experiments have shown how older adults heavily rely on the context of the conversation to compensate for their speech-comprehension problems (Lash et al. 2013; Pichora-Fuller et al. 1995; Rogers et al. 2012; Rogers and Wingfield 2015; Tun et al. 2002). Interestingly, results from Tun et al. (2002) and Brouwer et al. (2012) suggest that the type of background noise (meaningful vs. meaningless) could play a key role in the way that older adults process speech. Critically, the results from the Tun et al. (2002) study revealed how having a meaningful distractor (comprehensible words spoken in English) impaired the understanding of the target speech to a greater extent than a meaningless distractor (incomprehensible words spoken in Dutch) in older but not in younger adults. It should be noted that despite evidence in favor of older adults making greater use of semantic context than younger adults (Pichora-Fuller et al. 1995), some studies have suggested that these differences are due to a ceiling effect (Dubno et al. 2000).
Another important aspect in speech processing is the additional cognitive demand imposed by degraded stimuli that leads older adults to allocate more resources, such as attention, which would otherwise be available for secondary tasks (Anderson Gosselin and Gagne 2011; Tun et al. 2009; Ward et al. 2016). Cognitive processes, such as attention and inhibitory control, are indeed critical for understanding speech in noise. Several studies have shown that individuals who perform well on cognitive tasks that measure attention and inhibition tend to be less distracted by competing talkers and to be more efficient in focusing on the target speech signal (Colflesh and Conway 2007; Conway et al. 2001). Both inhibitory control and attention can be measured with a Flanker task (Weintraub et al. 2013).
Altogether, these studies suggest not only that speech-in-noise performance is regulated by a combination of bottom-up and top-down processes that contribute to efficient stream segregation but also that informational content of noise might have a different impact on the segregation of one auditory stream from another in the cortex, where brain plasticity plays a critical role in building the final representation of the attended sound stream.
The concept of brain plasticity is particularly important when performing electrophysiological measurements to study the processing of the auditory stimulus at different levels in the auditory system. A recent study has demonstrated a central compensatory gain mechanism strong enough to restore the representation of sounds at the cortical level in cases of absent auditory brain stem responses induced by auditory neuropathy (Chambers et al. 2016). Critically, attention-related brain plasticity has been consistently observed in the cortex in both animal and human experiments (Bidet-Caulet et al. 2007; Choi et al. 2013; Fritz et al. 2003; Lee and Middlebrooks 2011) but less reliably in the midbrain. It is well known that corticofugal projections from the cortex to midbrain have the ability to regulate and change the activity in lower nuclei (Suga 2008). However, their influence on the kind of short-time plasticity modulated by behavioral tasks, such as attentional tasks, is not well understood. Some recent results bring evidence against the possibility that responses at such a low auditory level might be controlled by higher cognitive processes (Varghese et al. 2015), whereas others suggest that there is evidence for the existence of this specific task-related plasticity (Slee and David 2015).
Results from Presacco et al. (2016) demonstrate that both midbrain and cortical responses were degraded in older adults to a greater extent than in younger adults, particularly for the most challenging listening conditions. The current study investigates the differing neural mechanisms underlying age-related deficits in speech-in-noise understanding arising from different types of noise (meaningful vs. meaningless) and at different signal-to-noise ratios (SNRs) in the midbrain and in the cortex. EEG was used to record frequency following responses (FFRs) recorded from the midbrain, as this neuroimaging technique is sensitive to subcortical activity and has a resolution on the order of milliseconds. Magnetoencephalography (MEG) was used to record cortical data, because magnetic fields are not volume conducted and thus pass undistorted through the scalp. MEG was not deemed appropriate for midbrain analysis because of its insensitivity to subcortical activity [Hämäläinen et al. (1993), although see Coffey et al. (2016)].
We posit several hypotheses. First, in midbrain responses, we hypothesize that different SNRs, but not different informational content of noise, will significantly affect the fidelity of the response of younger and older adults. The latter hypothesis stems from evidence that FFRs are not affected by the attentional state of individual participants (Varghese et al. 2015). Conversely, in the cortex, we expect the reconstruction fidelity (that is, the ability of the brain to track the speech envelope) to be more measurably augmented by the use of meaningless noise in older rather than in younger adults. Finally, we hypothesize that a cognitive decline in older adults will be correlated with an increase in cortical reconstruction accuracy across participants, as several studies have shown age-related increases in amplitude (Alain et al. 2014; Soros et al. 2009). These suppositions stem from the cited studies and from the observation that the overly high reconstruction fidelity of the speech envelope observed for older adults in our previous study (Presacco et al. 2016) may be a biomarker representing both an imbalance between excitatory and inhibitory processes and an inefficient use of cognitive resources.
MATERIALS AND METHODS
Participants
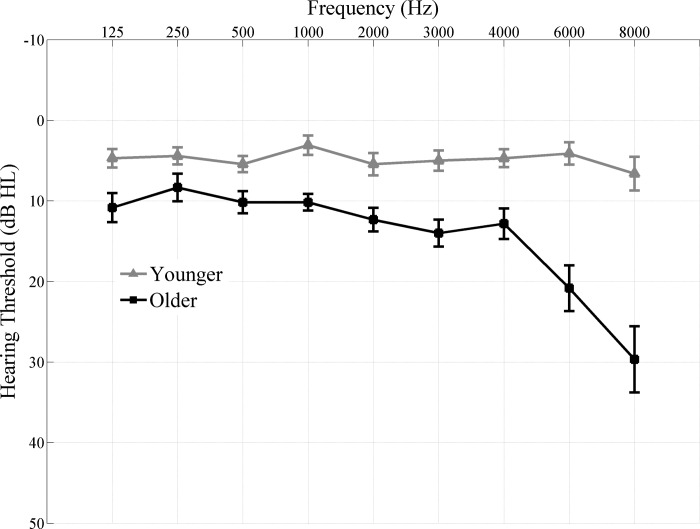
Participants comprised 17 younger adults (18–27 yr, means ± SD 22.23 ± 2.27, 3 men) and 15 older adults (61–73 yr old, means ± SD 65.06 ± 3.30, 5 men) recruited from the Maryland; Washington, D.C.; and Virginia areas. All procedures were reviewed and approved by the Institutional Review Board of the University of Maryland. Participants gave informed consent and were paid for their time. These participants were the same as those used in our previous study (Presacco et al. 2016), data for which were obtained during the same sessions as this study. To minimize the effects of audibility, only clinically normal-hearing listeners were included in both the younger and older age groups. All participants had clinically normal hearing (Fig. 1) defined as follows: 1) air conduction thresholds ≤25 dB hearing level (HL) from 125 to 4,000 Hz bilaterally and 2) no interaural asymmetry (>15 dB HL difference at no more than 2 adjacent frequencies). Participants had normal intelligence quotient scores [≥85 on the Wechsler Abbreviated Scale of Intelligence (Zhu and Garcia 1999)] and were not significantly different on intelligence quotient [F(1,30) = 0.660, P = 0.423] and sex (Fisher's exact, P = 0.423). In addition, the older adults were screened for dementia on the Montreal Cognitive Assessment (Nasreddine et al. 2005). The mean ± SD of the dementia screening was 26.9 ± 2.7. Our cutoff was 22, and all of our participants scored 22 or above. Because of the established effects of musicianship on subcortical auditory processing (Bidelman and Krishnan 2010; Parbery-Clark et al. 2012), professional musicians were excluded. All participants participated in both the EEG and MEG study, spoke English as their first language, and had no understanding of Dutch. Dutch was used as a masker because of its similarity to English in terms of phonological inventory and prosodic contours (Collier and Hart 1975). EEG and MEG data for each participant were collected in two separate sessions.
Fig. 1.
Audiogram (mean ± 1 SE) of the grand averages of younger (gray) and older (black) adults. All participants have clinically normal hearing (pure-tone thresholds ≤25 dB HL from 125 to 4,000 Hz).
Speech Intelligibility
The Quick Speech-in-Noise test (Killion et al. 2004) was used to quantify the ability to understand speech in noise composed of four-talker babble.
EEG: Stimuli and Recording
A 170-ms /da/ (Anderson et al. 2012) was synthesized at a 20-kHz sampling rate with a Klatt-based synthesizer (Klatt 1980). The stimulus was presented at 75 peak dB sound pressure level diotically with alternating polarities at a rate of 4 Hz through electromagnetically shielded insert earphones (ER·1; Etymotic Research, Elk Grove Village, IL) via Xonar Essence One (ASUS, Taipei, Taiwan) using Presentation software (Neurobehavioral Systems, Berkeley, CA). FFRs were recorded in quiet and in four noise levels: +3, 0, −3, and −6 dB SNR, defined as the root mean square (RMS) values between the speech syllable /da/ and the single female competing talker used as the background noise. Each of these four SNR noise levels was presented with meaningful (female native English speaker narrating A Christmas Carol by Charles Dickens) and meaningless (female native Dutch speaker narrating Aljaska en de Canada-spoorweg by Anonymous) background noise. The EEG data were recorded at a sampling frequency of 16,384 Hz using the ActiABR-200 acquisition system (BioSemi B.V., Amsterdam, Netherlands) with a standard vertical montage of five electrodes (Cz active, forehead ground common mode sense/driven right leg electrodes, earlobe references) and with an online, 100- to 3,000-Hz filter. During the recording session (∼2 h), participants sat in a recliner and watched a silent, captioned movie of their choice to facilitate a relaxed yet wakeful state. Two thousand artifact-free sweeps were recorded for each of the nine total noise levels from each participant.
Data Analysis
Data recorded with BioSemi were analyzed in MATLAB (version R2011b; MathWorks, Natick, MA) after being converted into MATLAB format with the function pop_biosig from EEGLab (Delorme and Makeig 2004). Sweeps with amplitude in the ±30-μV range were retained and averaged in real time and then processed offline using MATLAB (version R2011b; MathWorks). The time window for each sweep was −47 to 189 ms referenced to the stimulus onset. Responses were digitally bandpass filtered offline from 70 to 2,000 Hz using a fourth-order Butterworth filter to minimize the effects of cortical low-frequency oscillations. A final average response was created by averaging the sweeps of both polarities to minimize the influence of cochlear microphonic and a stimulus artifact on the response and simultaneously to maximize the envelope response (Aiken and Picton 2008; Campbell et al. 2012; Gorga et al. 1985). RMS values were calculated for the transition (18–68 ms) and steady-state (68–170 ms) regions. Correlation (Pearson's linear correlation) between the envelope response in quiet and noise was calculated for each participant to estimate the extent to which noise affects the FFR. Pearson's linear correlation was also used to quantify the stimulus-to-response correlation in the steady-state region, during which, the response may more reliably follow the stimulus. For this analysis, the envelope of the analytic signal of the stimulus was extracted and then band-pass filtered using the same filter as for the response.
MEG: Stimuli and Recording
The same participants recruited for the EEG study participated in the MEG experiment. Participants were asked to attend to one of two stories (foreground) presented diotically while ignoring the other one. The stimuli for the foreground consist of narrated segments from the book, The Legend of Sleepy Hollow by Washington Irving. The stimuli for the background were the same used in the EEG experiment. The foreground was spoken by a male talker, whereas the background story was spoken by a female talker. Each speech mixture was constructed as described by Ding and Simon (2012) by digitally mixing two speech segments into a single channel with a duration of 1 min. Five different SNR levels, presented to each participant in randomized order, were recorded: quiet, +3, 0, −3, and −6 dB SNR. The condition in quiet was recorded with two different segments. At each SNR level, two segments (1 from the foreground and 1 from the background) were played diotically. As also in the case of the EEG part of this study, the four noise levels were presented in two different scenarios: meaningful noise (where the competing talker was a female native English speaker narrating the story in English) and meaningless noise (where the competing talker was a female native Dutch speaker narrating the story in Dutch). The male speaker was always used as the foreground speaker, and eight different segments from the same story were used to minimize the possibility that the clarity of the stories could affect the performance of the participants. Three trials of each noise level were presented for a total of 30 trials (8 noise levels × 3 trials = 24 trials in noise and 2 speech segments × 3 trials = 6 trials in quiet). To maximize the level of attention of the participant on the foreground segment, participants were asked beforehand to count silently the number of times a specific word or name was mentioned in the story. The sounds (∼70 dB sound pressure level when presented with a solo speaker) were delivered to the participants' ears with 50 Ω sound tubing (E-A-RTONE 3A; Etymotic Research), attached to E-A-RLINK foam plugs inserted into the ear canal. The entire acoustic delivery system was equalized to give an approximately flat transfer function from 40 to 3,000 Hz, thereby encompassing the range of the presently delivered stimuli. Neuromagnetic signals were recorded using a 157-sensor whole-head MEG system (Kanazawa Institute of Technology, Nonoichi Ishikawa, Japan) in a magnetically shielded room, as described in Ding and Simon (2012).
Data Analysis
Three reference channels were used to measure and cancel the environmental magnetic field by using time shift-principal component analysis (de Cheveigné and Simon 2007). MEG data were analyzed offline using MATLAB. The 157 raw MEG data channel responses were first filtered between 2 and 8 Hz, with an order 700 windowed (Hamming) linear-phase finite impulse response filter, and then decomposed into N signal components (where N ≤ 157) using the denoising source separation (DSS) algorithm (de Cheveigné and Simon 2008; Särelä and Valpola 2005). The first six DSS component filters were then used for the analysis. The filtering range of 2–8 Hz was chosen based on previous results, showing the absence of intertrial coherence above 8 Hz (Ding and Simon 2013) and the importance of the integrity of the modulation spectrum above 1 Hz to understand spoken language (Greenberg and Takayuki 2004). The signal components used for analysis were then re-extracted from the raw data for each trial, spatially filtered using the six DSS filters just constructed, band-pass filtered between 1 and 8 Hz (Ding and Simon 2012) with a second-order Butterworth filter, and averaged over trials. Reconstruction of the envelope was performed using a linear reconstruction matrix estimated via the Boosting algorithm (David et al. 2007; Ding et al. 2013; Ding and Simon 2013). Success of the reconstruction is measured by the linear correlation between the reconstructed and actual speech envelope. The reconstructed envelope was obtained only from the speech of the single speaker alone to which the participant was instructed to attend, not of the actual mixed acoustic stimulus. The envelope was computed as the 1- to 8-Hz band-pass filtered magnitude of the analytic signal. To optimize the reconstruction fidelity, data were analyzed in a 500-ms integration window (Ding and Simon 2013; Presacco et al. 2016). The noise floor was calculated by using the neural response recorded from each noise level tested to reconstruct the speech envelope of a different stimulus than was used during this response. The different stimulus used was a 1-min speech segment extracted from a different story than that used for this experiment, which allows the noise floor to incorporate contributions from potential overfitting.
Cognitive Test
The Flanker Inhibitory Control and Attention Test of the National Institutes of Health Toolbox was used to measure executive function (ability to inhibit visual attention to irrelevant tasks) and attention (National Institutes of Health 2013). Participants were shown a series of arrows and were asked to determine, as quickly as possible, the direction of the middle arrow by pressing the space bar. The unadjusted scale score was used to compare age-related differences. The Conners Continuous Auditory Test of Attention (Multi-Health Systems, North Tonawanda, NY) was also used to assess attention, but since no significant differences were found between the two age groups, the results are not further discussed in this manuscript.
Statistical Analyses
All statistical analyses were conducted in SPSS version 21.0 (IBM, Armonk, NY). Fisher's z transformation was applied to all of the correlation values calculated for the midbrain and MEG analysis before running statistical analyses. Repeated-measures (RM) ANOVAs were performed with two within-participant independent variables (noise, 4 levels: +3, 0, −3, and −6 dB SNR; type of noise, 2 levels: meaningful and meaningless) in both MEG and FFR data. Split-plot ANOVAs were used to test for age group × noise type interactions for the RMS values of the FFR response in the time domain and for correlation values calculated for the MEG data. The Greenhouse-Geisser test was used when the Mauchly's sphericity test was violated. A paired t-test was used for within-participant group analysis for the RMS value of the amplitude of the FFR, for correlation values of the FFR, and for the MEG data. One-way ANOVAs were used to analyze the RMS values of the amplitude of the FFR and the correlation values of the FFR and for the MEG data. The nonparametric Mann-Whitney U-test was used in place of one-way ANOVA when Levene's test of Equality of Variances was violated. A one-sample t-test was used to evaluate the slope across noise levels of the RMS calculated for the four noise levels in the transition region. Two-tailed Spearman's rank correlation (ρ) was used to evaluate the relationships between cognitive score and midbrain and cortical parameters. The false discovery rate procedure (Benjamini and Hochberg 1995) was applied to control for multiple comparisons where appropriate.
RESULTS
Speech Intelligibility
Younger adults (means ± SD = −0.573 ± 1.13 dB SNR loss) scored significantly better [F(1,30) = 10.613, P = 0.003] than older adults (means ± SD = 0.8 ± 1.25 dB SNR loss), suggesting that older adults' performance in noise may decline compared with younger adults, even when audiometric thresholds are clinically normal.
Midbrain (EEG)
Amplitude analysis.
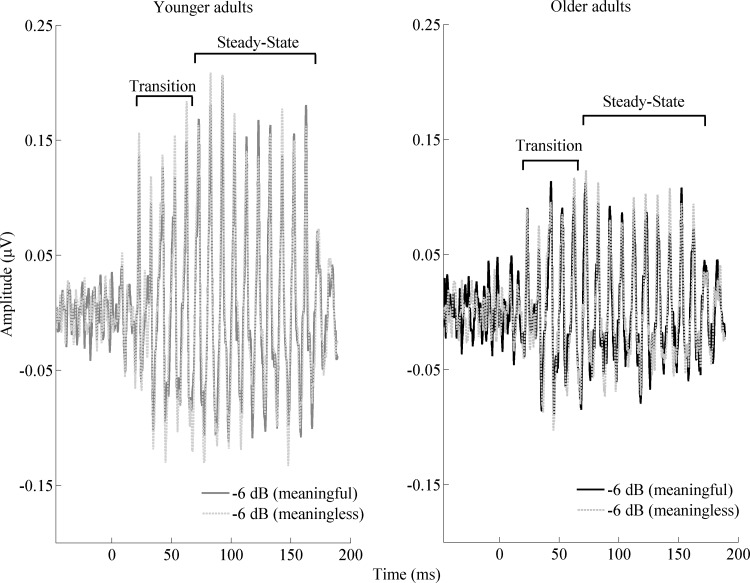
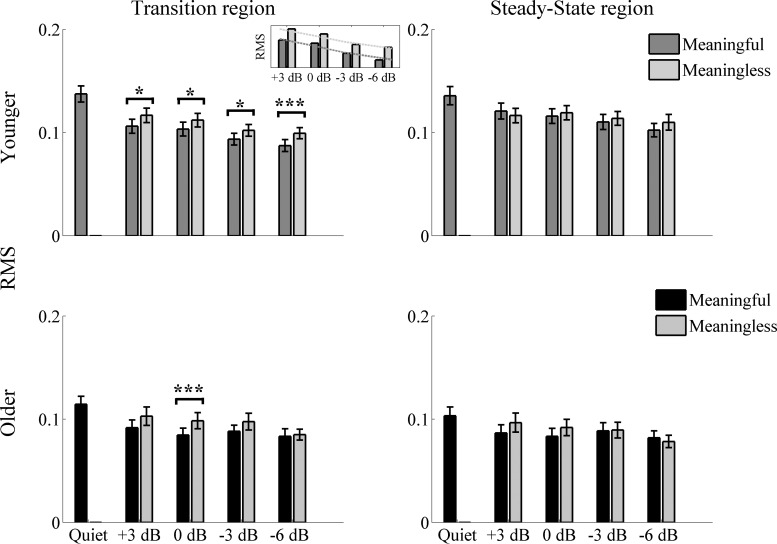
Figure 2 shows the grand average of FFRs to the stimulus envelope of younger and older adults in quiet and noise. The ability of midbrain neurons to synchronize in response to the stimulus was assessed by measuring the strength of the FFR response via its RMS value. Overall results show a stronger response in younger adults in both the transition and steady-state regions. The effect of the type of noise was only seen consistently in younger adults and only in the transition region. Older adults' responses are not degraded by the more-challenging noise levels, possibly because their responses are already degraded, even in quiet. Figure 3 displays the RMS values of younger and older adults for each SNR level tested with meaningful and meaningless noise.
Fig. 2.
Grand averages (n = 17 for younger and n = 15 for older adults) of FFRs to the stimulus envelope for younger [left; meaningful noise (−6 dB; dark gray); meaningless noise (−6 dB; light gray)] and older [right; meaningful noise (−6 dB; black); meaningless noise (−6 dB; gray)] adults.
Fig. 3.
RMS values ± 1 SE for younger (top) and older (bottom) adults in the transition (left) and steady-state (right) regions for all of the noise levels tested. Different informational content of noise affects only the transition response and mainly in younger adults. A significant effect of the different informational content of noise was seen only in the transition region in all of the noise levels tested in younger adults (P < 0.05 at +3, 0, and −3 dB, and P < 0.001 at −6 dB) and only at 0 dB in older adults (P < 0.001). Significantly higher RMS values in younger adults than in older adults in all of the noise levels tested were found only in the steady-state region. Inset: for the transition region in younger adults, separate linear fits to the RMS for both types of noise reveal a 4.6-dB SNR (horizontal shift between the dashed best-fit lines) advantage for meaningless noise over meaningful. *P < 0.05, ***P < 0.001.
transition region.
RM ANOVA showed no noise level × noise type × age interaction [F(3,90) = 1.559, P = 0.214] and no noise level × noise type interaction [F(3,90) = 0.586, P = 0.626]. A paired t-test showed significantly higher RMS values with meaningless noise in both younger [t(16) = −2.911, P = 0.01; t(16) = −2.234, P = 0.04; t(16) = −2.522, P = 0.02; and t(16) = −4.283, P < 0.001, for +3; 0; −3; and −6 dB, respectively] and older adults only at 0 dB [t(14) = −4.514, P < 0.001]. A regression analysis was also carried out to fit the noise levels. A one-sample t-test showed that the slopes for both meaningful and meaningless noise are significantly different from zero in younger adults [t(16) = 4.763, P < 0.001 and t(16) = 4.247, P < 0.001, for meaningful and meaningless noise, respectively], whereas in older adults, only the slopes of the meaningless noise were significantly different from zero [t(14) = 0.886, P = 0.391 and t(16) = 3.910, P = 0.002, for meaningful and meaningless noise, respectively]. Additionally, the regression analysis revealed a 4.6-dB neural advantage in younger adults for meaningless noise over meaningful noise (horizontal shift in Fig. 3). Given these results, we tested whether an age × noise interaction was present to investigate if the different slopes observed in older adults translated also in different ways of encoding the speech syllable /da/ at different noise levels. RM ANOVA showed no noise type × age interaction in any of the noise levels tested (all P values > corrected significance threshold). RM ANOVA showed a noise level × age interaction between quiet and noise at −3 dB [F(1,30) = 6.264, P = 0.018] and −6 dB [F(1,30) = 6.696, P = 0.015] but not at the other noise levels tested with meaningful noise [F(1,30) = 1.125, P = 0.297 and F(1,30) = 0.333, P = 0.568, for +3 and 0 dB, respectively]. Conversely, with meaningless noise, the interaction was found at −3 dB [F(1,30) = 8.097, P = 0.008] but not at +3 dB [F(1,30) = 1.294, P = 0.264], 0 dB [F(1,30) = 1.986, P = 0.169], and −6 dB [F(1,30) = 1.784, P = 0.192]. A one-way analysis of covariance [using the condition in quiet as covariate, as younger adults have significantly higher RMS values, F(1,30) = 4.255, P = 0.048] was used to evaluate the strength of the response in noise between the two age groups. Results showed no significant differences between younger and older adults in any of the noise levels tested with meaningful noise [F(1,29) = 0.007, P = 0.936; F(1,29) = 0.296, P = 0.590; F(1,29) = 1.941, P = 0.174; and F(1,29) = 2.511, P = 0.124, for +3; 0; −3; and −6 dB, respectively] and meaningless noise [F(1,29) = 0.195, P = 0.662; F(1,29) = 0.278, P = 0.602; F(1,29) = 3.779, P = 0.062; and F(1,29) = 0.077, P = 0.783, for +3; 0; −3; and −6 dB, respectively].
steady-state region.
RM ANOVA showed a noise level × noise type × age interaction [F(3,90) = 4.376, P = 0.006] but no noise level × noise type interaction [F(3,90) = 0.508, P = 0.678]. A follow-up one-way ANOVA showed a noise type × age interaction only at +3 dB [F(1,30) = 10.443, P = 0.003; F(1,30) = 0.945, P = 0.339; F(1,30) = 0.173, P = 0.681; and F(1,30) = 4.818, P = 0.036, for +3; 0; −3; and −6 dB, respectively]. A paired t-test showed no significant differences between meaningful and meaningless noise in either age group (all P values > corrected significance threshold). RM ANOVAs also show no noise type × age interaction among quiet and the two types of noise tested (all P values > corrected significance threshold). Given these results, the RMS for meaningful and meaningless noise was collapsed together in one single analysis. A one-way ANOVA showed significantly higher RMS value in younger adults than in older adults in all of the noise levels tested [F(1,62) = 11.632, P = 0.001; F(1,62) = 16.606, P < 0.001; F(1,62) = 9.813, P = 0.003; and F(1,62) = 14.840, P < 0.001, for +3; 0; −3; and −6 dB, respectively].
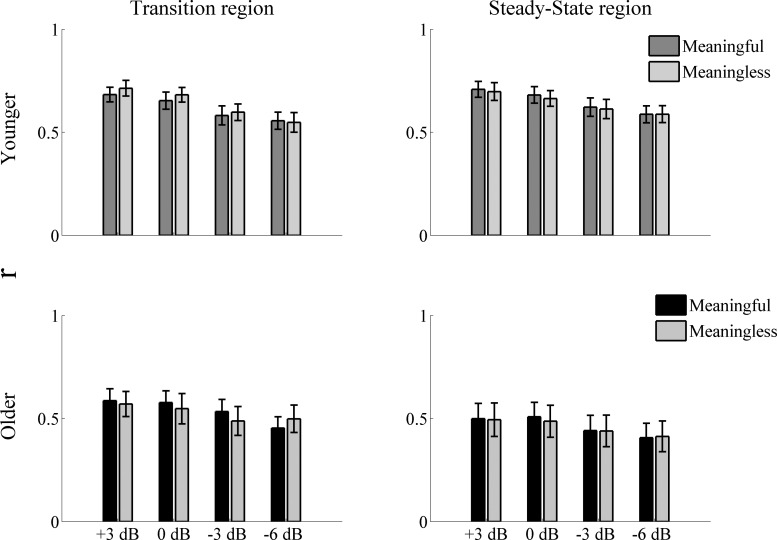
Correlation analysis.
To analyze the robustness of the response profile in noise (the ability of the midbrain neurons to fire similarly in quiet and in noise), we linearly correlated (Pearson correlation) the average response (Fig. 4) obtained in quiet with the ones obtained in noise for both the transition and steady-state regions for each participant. Results show no differences between the type of noise in either age group and higher correlations in younger adults. RM ANOVA showed no noise level × noise type × age interaction in either the transition [F(3,90) = 2.521, P = 0.063] or the steady-state [F(3,90) = 0.013, P = 0.998] region and no noise level × noise type interaction in either the transition [F(3,90) = 0.783, P = 0.506] or the steady-state [F(3,90) = 0.425, P = 0.735] region. RM ANOVA showed no noise type × age interaction (all P values > 0.05) between meaningful and meaningless noise in any of the noise levels tested in either region. A paired t-test also showed no significant differences between meaningful and meaningless noise in any of the noise levels tested (all P values > corrected significance threshold) in either region. Given these results, the correlation values for meaningful and meaningless noise were collapsed together for further analyses for both the transition and the steady-state regions. The Mann-Whitney U-test was used to study the differences between groups. Older adults were not significantly more affected by noise levels than younger adults in the transition condition, as revealed by no significant differences found in the transition region in any of the noise levels tested [U(62) = 398, Z = −1.507, P = 0.132; U(62) = 424, Z = −1.157, P = 0.247; U(62) = 439, Z = −0.955, P = 0.339; and U(62) = 442, Z = −1.184, P = 0.236, for +3; 0; −3; and −6 dB, respectively]. Conversely, in the steady-state region, older adults were more affected by noise level than younger adults, as suggested by significantly higher r values found in younger adults at all of the noise levels tested [U(62) = 314, Z = −2.637, P = 0.008; U(62) = 333, Z = −2.381, P = 0.017; U(62) = 349, Z = −2.166, P = 0.03; and U(62) = 329, Z = −2.435, P = 0.015, for +3; 0; −3; and −6 dB, respectively].
Fig. 4.
Pearson correlation coefficient ± 1 SE of the quiet-to-noise correlation for younger (top) and older (bottom) adults in the transition (left) and steady-state (right) regions for all of the noise levels tested. Results showed no significant effect of the type of noise in either younger or older adults at any of the noise levels tested.
Stimulus-to-response correlation.
The correlation between stimulus and neural response was calculated to quantify the ability of the brain to follow the auditory input. Our results show higher correlations in younger adults, reflected by a significant noise level × age interaction. Specifically, RM ANOVA showed no noise level × noise type × age interaction [F(3,90) = 0.471, P = 0.703] and no noise level × noise type interaction [F(3,90) = 2.441, P = 0.069]. RM ANOVA showed no noise type × age interaction (all P values > 0.05) between meaningful and meaningless noise at any of the noise levels tested. RM ANOVA showed a significant noise level × age interaction between quiet and noise at all of the noise levels tested with meaningful noise [F(1,30) = 5.915, P = 0.021; F(1,30) = 4.302, P = 0.047; F(1,30) = 5.786, P = 0.023; F(1,30) = 8.318, P = 0.007, for +3; 0; −3; and −6 dB, respectively] and at all of the noise levels tested, except −3 dB, with meaningless noise [F(1,30) = 8.356, P = 0.007; F(1,30) = 6.269, P = 0.018; F(1,30) = 4.171, P = 0.05; and F(1,30) = 8.305, P = 0.007, for +3; 0; −3; and −6 dB, respectively]. A one-way ANOVA showed that the younger adults' correlation values were significantly higher than those of older adults at all of the noise levels tested with meaningful [F(1,30) = 7.768, P = 0.009; F(1,30) = 5.535, P = 0.025; F(1,30) = 5.166, P = 0.030; and F(1,30) = 8.838, P = 0.006, for +3; 0; −3; and −6 dB, respectively] and meaningless [F(1,30) = 8.414, P = 0.007; F(1,30) = 6.293, P = 0.013; F(1,30) = 5.031, P = 0.032; and F(1,30) = 9.290, P = 0.005, for +3; 0; −3; and −6 dB, respectively] noise. No significant differences were found in quiet [F(1,30) = 0.109, P = 0.744].
Cortex (MEG): Reconstruction of the Speech Envelope
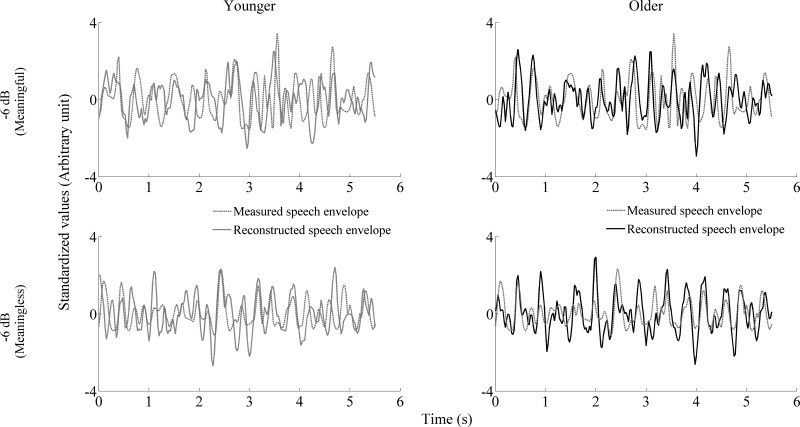
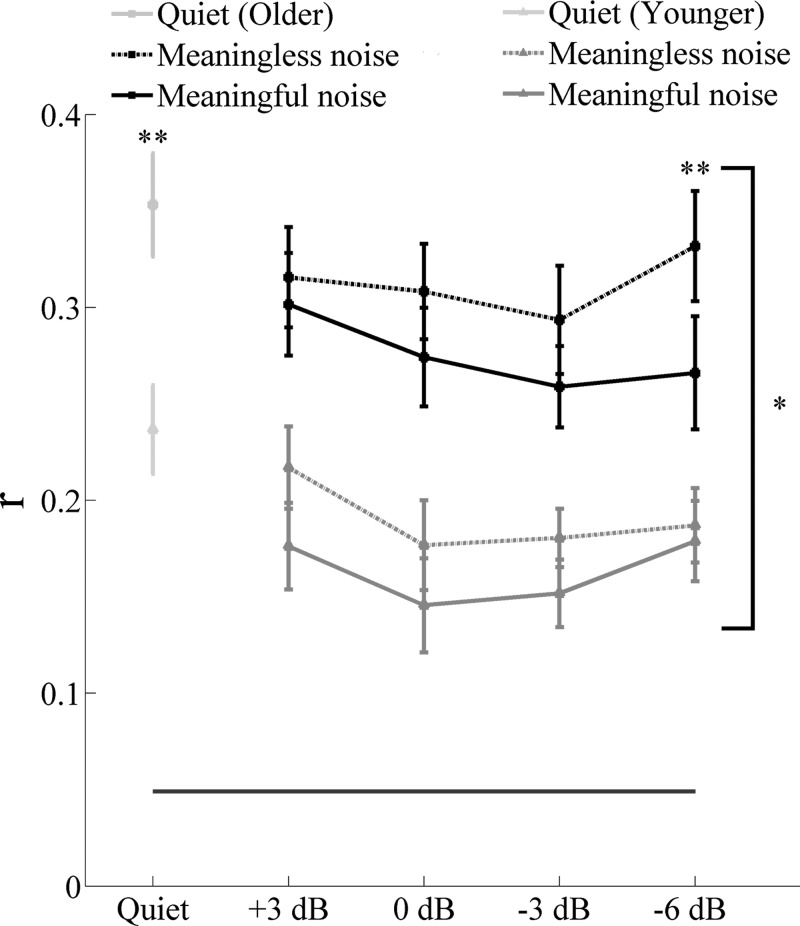
The ability to reconstruct the low-frequency speech envelope from cortical activity is a measure of the fidelity of the neural representation of that speech envelope (Ding and Simon 2012). Figure 5 shows an example of reconstruction of the speech envelope of the foreground for younger and older adults in noise (−6 dB). Figure 6 displays the grand average ± SE of the reconstruction accuracy for younger and older adults for all of the noise levels tested with meaningful and meaningless noise. Data recorded in quiet were from two different, 1-min excerpts. Since no significant interactions [F(1,30) = 2.340, P = 0.137] or differences between the two excerpts in quiet in both younger [t(16) = −0.078, P = 0.939] and older [t(14) = 1.776, P = 0.098] adults were found, their reconstruction accuracy values were averaged together for further analyses. An independent t-test showed significantly higher correlation values in quiet in older adults [t(1,30) = −3.272, P = 0.003]. RM ANOVA applied to all of the noise levels showed no noise level × noise type × age interaction [F(3,90) = 1.919, P = 0.132]. Results of an RM ANOVA applied to all of the noise levels showed a noise level [F(3,90) = 3.946, P = 0.011] and noise type effect [F(3,90) = 21.278, P < 0.001]. RM analysis of covariance (with correlation in quiet used as covariate) revealed that the noise type effect was driven by a type of noise × age interaction at −6 dB [F(1,29) = 7.008, P = 0.013] but not at the other noise levels tested [F(1,29) = 0.717, P = 0.404; F(1,29) = 0.010, P = 0.922; F(1,29) = 0.001, P = 0.976, for +3; 0; and −3 dB, respectively]. A follow-up t-test showed significant differences between meaningful and meaningless noise at −6 dB only in older adults [t(14) = −3.659, P = 0.003]. A paired t-test showed that reconstruction fidelity was significantly higher than the noise floor in both younger and older adults at all of the noise levels tested (all P values < 0.01). Despite a remarkable drop in reconstruction fidelity between quiet and noise in older adults, their correlation values were still significantly better than younger adults at all of the noise levels tested (all P < 0.05 with one-way ANOVA applied without covariate).
Fig. 5.
Example of the reconstruction of the speech envelope of the foreground for younger (left) and older (right) adults in noise (−6 dB). Top: the reconstructed envelope with meaningful noise; bottom: the reconstructed envelope for meaningless noise. The waveforms have been standardized for visualization purposes.
Fig. 6.
Reconstruction accuracy value ± 1 SE of the speech envelope of the foreground for younger and older adults in quiet and in meaningful and meaningless noise. The bottom horizontal line shows the noise floor. Older adults' reconstruction fidelity is significantly better than that of the younger adults at all of the noise levels tested. Only older adults show significant improvement at −6 dB when competing speech was meaningless noise. *P < 0.05, **P < 0.01.
Relationships Among Cognitive Test, Midbrain, and Cortical Data
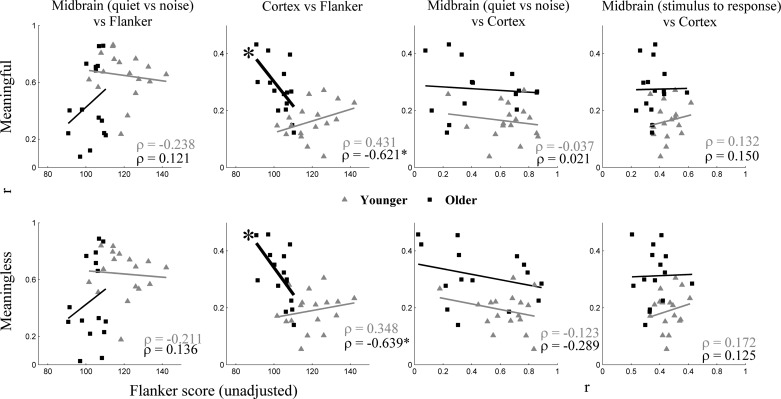
The Flanker Inhibitory Control and Attention test showed significantly higher scores for younger adults than for older adults [F(1,30) = 27.375, P < 0.001]. The Flanker score was evaluated with respect to the brain measures. Significant negative correlations (lower score associated with higher reconstruction accuracy) were found between the Flanker Inhibitory Control and Attention test score and the cortical response (average cortical decoding accuracy across all of the noise levels; ρ = −0.621, P = 0.013 and ρ = −0.639, P = 0.01, for meaningful and meaningless noise, respectively) in older but not in younger adults (ρ = 0.431, P = 0.084 and ρ = 0.348, P = 0.171, for meaningful and meaningless noise, respectively). No significant correlation was found between the Flanker Inhibitory Control and Attention test score and midbrain responses (average correlation across all of the noise levels between quiet and noise in the steady-state region and average correlation across all of the noise levels between stimulus and response) in either younger or older adults (all P > 0.05). Similarly, no significant correlations were found between midbrain and cortical responses in either younger or older adults (P > 0.05). Figure 7 shows the data for each participant.
Fig. 7.
Scatter plots of several neural and cognitive statistics for each participant tested: Flanker score (cognitive; left 2 columns), midbrain quiet-to-noise correlation (first and third columns), midbrain stimulus-to-response correlation in noise in the steady-state region (right 2 columns), and cortex reconstruction accuracy values (right 3 columns) in both meaningful (top) and meaningless (bottom) noise. Significant negative correlations were found between Flanker score and cortical reconstruction accuracy values only in older adults (second column, black squares only).
DISCUSSION
The results of this study provide support for most, but not all, of our initial hypotheses. Behavioral data showed that older adults do have poorer speech understanding in noise than younger adults, despite their normal audiometric hearing thresholds. Unexpectedly, younger adults' midbrain responses are less affected by meaningless rather than meaningful noise in the transition region, an effect not seen in older adults. Consistent with our previous study (Presacco et al. 2016), the fidelity of the reconstruction of speech in cortex remains higher in older than in younger adults, even with meaningless noise. Interestingly, in the most challenging noise level (−6 dB), the different effects of meaningful and meaningless noise were only seen in older adults. This is in contrast with the findings seen in FFR recordings from older adults, where no significant differences were seen between meaningful and meaningless noise, likely because of a more robust top-down processing in younger adults. Cortical reconstruction results were also significantly correlated with cognitive scores in older adults, as hypothesized, in that the higher reconstruction accuracy, the lower their cognitive score.
Midbrain
Amplitude response.
transition region.
Contrary to what might have been expected, younger adults showed significant differences in the midbrain for all noise levels tested when meaningless vs. meaningful noise was played in the background, suggesting a substantial effect of top-down mechanisms in the young midbrain. The amount of influence of higher-level cognitive processes on the midbrain has led to varying results in the literature, some showing strong effects of attention (Slee and David 2015), with others failing to find any attentional-related change (Varghese et al. 2015). In our study, participants were asked to listen passively to auditory stimuli while watching a silent movie, which should minimize any potential effects of attention. However, it is possible that the midbrain could have encoded FFR in different ways due to the different natures of the background noise. Another potential explanation, following the results of Coffey et al. (2016), would be that the FFR is influenced by contributions from the cortex. However, this explanation is not probable, given that the effect was specific to the transition, and the early region of the response is less likely to have a cortical origin. Interestingly, the neural advantage estimated for meaningless noise over meaningless noise was ∼4.6 dB across all of the noise levels tested. The same level of effect was not observed in older adults (only significant at 0 dB), possibly due to their problems in encoding the high-frequency burst of the stimulus /da/, as reflected by significant RMS × age group interactions found in the two most challenging noise levels in the transition region, consistent with our previous studies (Presacco et al. 2015, 2016). This high-frequency burst could have potentially impaired the older adults' ability to encode correctly the transition region of the /da/, given their hearing loss at frequencies higher than 4 kHz. Whereas this impairment seems to be present already in quiet, the addition of noise, particularly at negative SNRs, may exacerbate its effect. Critically, older adults' slope of the line that best fits the RMS noise levels of meaningful noise is not significantly different from zero, suggesting that their midbrain response in this noise type is not dependent on SNR. This lowered response (compared with younger listeners) and the weak differences in response between the noise levels contribute additional evidence of temporal deficits in the transition region. It might be argued that these results reflect the difference in audiometric hearing thresholds measured in younger and older adults and that RMS in quiet is a proxy for hearing loss. However, we believe that this is not the case, as suggested by recent findings (Anderson et al. 2012) that show significant differences in RMS values even between age groups with equivalent hearing thresholds.
steady-state region.
In contrast, no noise type effect was found in the steady-state region for either age group.
Robustness of the Envelope to Noise
Differently from what we observed with the amplitude analysis, no significant differences in quiet-to-noise correlations were found for noise backgrounds of different informational content in younger adults in the transition region. The correlation analysis also supported the initial hypothesis that younger adults' responses should be more robust to noise than those of older adults and that the type of noise would have no effect on the response consistency. Younger adults showed significantly higher correlations at all of the noise levels tested in the steady-state region only, reinforcing the existence of a disruption of periodicity in the encoded speech envelope in older adults (Anderson et al. 2012; Mamo et al. 2016; Pichora-Fuller et al. 2007; Presacco et al. 2015, 2016). Additionally, the higher robustness of the envelope to noise in younger adults is confirmed by the results of the stimulus-to-response correlation, which shows that the ability of older adults' responses to follow the stimulus is significantly worse than that of younger adults in noise.
Cortex
Reconstruction of the speech envelope.
A critical part of our experiment was to investigate how different informational content of noise affects the ability to reconstruct the speech envelope of the attended speaker. As hypothesized, reconstruction accuracy was higher for both age groups in the presence of meaningless noise compared with meaningful noise at all SNRs; however, as the SNR decreased, older adults relied more on the type of background than younger adults to process speech, as revealed by a significant correlation × age interaction at −6 dB. These observations are in agreement with a previous study in which older and younger adults were challenged to recall target words in the presence of a meaningful (English) and meaningless (Dutch) distractor (Tun et al. 2002). Consistent with our results, whereas younger adults' reconstruction accuracy did not significantly differ between the two noise types, older adults' reconstruction accuracy was significantly enhanced when meaningless noise was used as distractor. At this point, we cannot rule out the possibility that this neural enhancement could also be partially driven by talker differences that could have affected, to a higher degree, older rather than younger adults.
The results of the reconstruction of the speech envelope, regardless of informational content, also showed an enhanced reconstruction in older adults, which is consistent with studies showing an exaggerated representation of cortical responses in older adults, both with and without hearing loss (Alain et al. 2014; Lister et al. 2011; Presacco et al. 2016; Soros et al. 2009; Tremblay et al. 2003). As discussed by Presacco et al. (2016), this over-representation of the response to speech (even in quiet) may result from a processing deficit or imbalance between excitatory and inhibitory mechanisms. Cognitive resource use may also play a role. Peelle et al. (2010) argue that aging specifically affects the efficient use of cognitive resources because of decreased cortical network connectivity. This, in turn, would cause neighboring cortical areas to process the same stimulus independently, instead of collaboratively, which could also lead to over-representation. Furthermore, several studies have suggested that aging might alter the balance between inhibitory and excitatory neural mechanisms in the cortex (de Villers-Sidani et al. 2010; Hughes et al. 2010; Juarez-Salinas et al. 2010; Overton and Recanzone 2016), which in turn, might lead to a stronger cortical response. The addition of a competing talker caused a substantial drop of decoding accuracy in older adults. This is consistent with recent results (Getzmann et al. 2016; Getzmann and Naatanen 2015) showing age-related changes in event-related potentials, recorded in a simulated “cocktail party” scenario, that could help explain the difficulties experienced by older adults in effectively segregating and encoding speech streams in noise conditions.
Relationships Among Cognitive, Midbrain, and Cortical Data
In our prior study (Presacco et al. 2016), one of our open questions was the role of previously reported age-related cognitive decline (Anderson Gosselin and Gagne 2011; Pichora-Fuller et al. 1995; Surprenant 2007; Tun et al. 2009) in explaining the over-representation of the cortical response. Here, we address this issue by analyzing the correlation between cognitive task and cortical response. Results from this analysis showed that older adults' cognitive decline in executive function is associated with higher speech envelope reconstruction. This negative correlation supports our hypothesis that higher reconstruction accuracy is not beneficial for older adults, but it is more likely the result of an abnormal increase in neural currents, perhaps caused by an imbalance between excitatory and inhibitory mechanisms. The failure of these mechanisms might explain the need for older adults to use supplementary cognitive resources to complete the task, characterized by activation of larger areas of the brains, including the prefrontal cortex (Wong et al. 2010) and the cingulo-opercular network (Vaden et al. 2015). Additionally, reduced coherence among brain regions involved in speech comprehension might also lead to inefficient use of these cognitive resources, making the speech-in-noise task even more challenging to accomplish (Peelle et al. 2010). Interestingly, younger adults showed a positive correlation between speech envelope reconstruction and cognitive score. Although this correlation was not significant, it suggests that these results further emphasize the uniqueness of older adults' findings, where bigger responses translate into worse behavioral performance. It is possible that in a young and healthy auditory system where a balance between excitatory and inhibitory process is still in place, a better reconstruction accuracy would translate into better behavioral performance (Ding and Simon 2013). Conversely, in an aging brain, an apparently “better” (bigger) response might actually result from overexcitation or a lack of efficient communication among brain regions, as discussed above.
Our analyses failed to find any correlation between subcortical and cortical measurements. This null result could be due to the different natures of the task and stimuli used to elicit FFR and cortical responses (Presacco et al. 2016). The use of different stimuli was motivated by the differing number of trials required by each brain area to obtain a clear neural signal. For the cortical analysis, three runs were sufficient to obtain an optimal response above the noise floor, whereas in the midbrain, a minimum of 2,000 runs was needed, making the use of stimuli longer than 170 ms not feasible for this long experiment. Additionally, it is entirely possible that before reaching the cortex, the auditory encoding of the target is further modified by other auditory areas, such as the thalamus, which could explain the lack of correlation between our measurements.
Although all of these explanations are plausible, however, we want to emphasize the possibility that the absence of correlation between midbrain and cortical measurement offers additional support to the hypothesis that compensatory central gain increases help restore the representation of an auditory stimulus at the cortical level, even in the absence of an auditory brain stem response (Chambers et al. 2016). These results would also suggest that this central gain mechanism is restricted in what it can accomplish, e.g., recovery of spike-rate encoding but not the encoding of precise spike timing.
Concluding Remarks
The overall results of our study give compelling support to our hypotheses of the existence of an age-related effect for different informational content of noise on the auditory response. These findings suggest that the type of noise could play a more critical role in speech-processing ability in older rather than in younger adults. The presence of meaningless noise led to significantly enhanced representation of the cortical response only in older adults. The strong correlation between cognitive decline and over-representation of the cortical response in older adults reinforces our hypothesis that larger cortical responses are not a biomarker that represents an advantageous response of the brain but rather, an abnormally high neural activity that could be an indicator of a failure in processing auditory information.
GRANTS
Funding for this study was provided by the University of Maryland College Park (UMCP) Department of Hearing and Speech Sciences, UMCP ADVANCE Program for Inclusive Excellence (NSF HRD1008117), and National Institute on Deafness and Other Communication Disorders (R01DC008342, R01DC014085, and T32DC-00046).
DISCLOSURES
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
A.P., J.Z.S., and S.A. conception and design of research; A.P. performed experiments; A.P. analyzed data; A.P., J.Z.S., and S.A. interpreted results of experiments; A.P. and S.A. prepared figures; A.P., J.Z.S., and S.A. drafted manuscript; A.P., J.Z.S., and S.A. edited and revised manuscript; A.P., J.Z.S., and S.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Natalia Lapinskaya for excellent technical support and to Dr. Sandra Gordon-Salant for her helpful comments on our manuscript.
REFERENCES
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hear Res 245: 35–47, 2008. [DOI] [PubMed] [Google Scholar]
- Alain C, Roye A, Salloum C. Effects of age-related hearing loss and background noise on neuromagnetic activity from auditory cortex. Front Syst Neurosci 8: 8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Drehobl S, Kraus N. Effects of hearing loss on the subcortical representation of speech cues. J Acoust Soc Am 133: 3030–3038, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci 32: 14156–14164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Kraus N. Neural timing is linked to speech perception in noise. J Neurosci 30: 4922–4926, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson Gosselin P, Gagne JP. Older adults expend more listening effort than young adults recognizing speech in noise. J Speech Lang Hear Res 54: 944–958, 2011. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57: 289–300, 1995. [Google Scholar]
- Bidelman GM, Krishnan A. Effects of reverberation on brainstem representation of speech in musicians and non-musicians. Brain Res 1355: 112–125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet-Caulet A, Fischer C, Bauchet F, Aguera PE, Bertrand O. Neural substrate of concurrent sound perception: direct electrophysiological recordings from human auditory cortex. Front Hum Neurosci 1: 5, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, McMillan GP, Penman TM, Gille SM. Predicting perception in noise using cortical auditory evoked potentials. J Assoc Res Otolaryngol 14: 891–903, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, Penman TM, McMillan GP, Ellis EM. Electrophysiology and perception of speech in noise in older listeners: effects of hearing impairment and age. Ear Hear 36: 710–722, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer S, Van Engen KJ, Calandruccio L, Bradlow AR. Linguistic contributions to speech-on-speech masking for native and non-native listeners: language familiarity and semantic content. J Acoust Soc Am 131: 1449–1464, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard RF, Sims D. A comparison of the effects of broadband masking noise on the auditory brainstem response in young and older adults. Am J Audiol 11: 13–22, 2002. [DOI] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Language and Aging, edited by Craik FI and Salthouse TA. New York: Psychology, 2008, p. 373–443. [Google Scholar]
- Campbell T, Kerlin JR, Bishop CW, Miller LM. Methods to eliminate stimulus transduction artifact from insert earphones during electroencephalography. Ear Hear 33: 144–150, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Schatteman TA, Turner JG. Age-related changes in the response properties of cartwheel cells in rat dorsal cochlear nucleus. Hear Res 216–217: 207–215, 2006. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol 30: 349–360, 1995. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci 25: 10952–10959, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers Anna R, Resnik J, Yuan Y, Whitton Jonathon P, Edge Albert S, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89: 867–879, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Rajaram S, Varghese LA, Shinn-Cunningham BG. Quantifying attentional modulation of auditory-evoked cortical responses from single-trial electroencephalography. Front Hum Neurosci 7: 115, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL. Aging degrades the neural encoding of simple and complex sounds in the human brainstem. J Am Acad Audiol 24: 590–599; quiz 643-594, 2013. [DOI] [PubMed] [Google Scholar]
- Coffey EB, Herholz SC, Chepesiuk AM, Baillet S, Zatorre RJ. Cortical contributions to the auditory frequency-following response revealed by MEG. Nat Commun 7: 11070, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colflesh GJ, Conway AR. Individual differences in working memory capacity and divided attention in dichotic listening. Psychon Bull Rev 14: 699–703, 2007. [DOI] [PubMed] [Google Scholar]
- Collier R, Hart JT. The role of intonation in speech perception. In: Structure and Process in Speech Perception: Proceedings of the Symposium on Dynamic Aspects of Speech Perception held at IPO, Eindhoven, Netherlands, August 4–6, 1975, edited by Cohen A, Nooteboom SG. Berlin, Heidelberg, Germany: Springer Berlin Heidelberg, 1975, p. 107–123. [Google Scholar]
- Conway AR, Cowan N, Bunting MF. The cocktail party phenomenon revisited: the importance of working memory capacity. Psychon Bull Rev 8: 331–335, 2001. [DOI] [PubMed] [Google Scholar]
- David SV, Mesgarani N, Shamma SA. Estimating sparse spectro-temporal receptive fields with natural stimuli. Network 18: 191–212, 2007. [DOI] [PubMed] [Google Scholar]
- de Cheveigné A, Simon JZ. Denoising based on spatial filtering. J Neurosci Methods 171: 331–339, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cheveigné A, Simon JZ. Denoising based on time-shift PCA. J Neurosci Methods 165: 297–305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RC, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci USA 107: 13900–13905, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Ding N, Chatterjee M, Simon JZ. Robust cortical entrainment to the speech envelope relies on the spectro-temporal fine structure. Neuroimage 88: 41–46, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Simon JZ. Adaptive temporal encoding leads to a background-insensitive cortical representation of speech. J Neurosci 33: 5728–5735, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Simon JZ. Emergence of neural encoding of auditory objects while listening to competing speakers. Proc Natl Acad Sci USA 109: 11854–11859, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno JR, Ahlstrom JB, Horwitz AR. Use of context by young and aged adults with normal hearing. J Acoust Soc Am 107: 538–546, 2000. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Aging and temporal discrimination in auditory sequences. J Acoust Soc Am 109: 2955–2963, 2001. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Auditory temporal processing in elderly listeners. J Am Acad Audiol 7: 183–189, 1996. [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res 106: 95–104, 1997. [DOI] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci 6: 1216–1223, 2003. [DOI] [PubMed] [Google Scholar]
- Getzmann S, Golob EJ, Wascher E. Focused and divided attention in a simulated cocktail-party situation: ERP evidence from younger and older adults. Neurobiol Aging 41: 138–149, 2016. [DOI] [PubMed] [Google Scholar]
- Getzmann S, Hanenberg C, Lewald J, Falkenstein M, Wascher E. Effects of age on electrophysiological correlates of speech processing in a dynamic “cocktail-party” situation. Front Neurosci 9: 341, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzmann S, Naatanen R. The mismatch negativity as a measure of auditory stream segregation in a simulated “cocktail-party” scenario: effect of age. Neurobiol Aging 36: 3029–3037, 2015. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Yeni-Komshian GH, Fitzgibbons PJ, Barrett J. Age-related differences in identification and discrimination of temporal cues in speech segments. J Acoust Soc Am 119: 2455–2466, 2006. [DOI] [PubMed] [Google Scholar]
- Gorga M, Abbas P, Worthington D. Stimulus calibration in ABR measurements. In: The Auditory Brainstem Response, edited by Jacobsen J. San Diego: College Hill, 1985, p. 49–62. [Google Scholar]
- Greenberg S, Takayuki A. What are the essential cues for understanding spoken language? IEICE Trans Inf Syst 87: 1059–1070, 2004. [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography-theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65: 413–497, 1993. [Google Scholar]
- He NJ, Mills JH, Ahlstrom JB, Dubno JR. Age-related differences in the temporal modulation transfer function with pure-tone carriers. J Acoust Soc Am 124: 3841–3849, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Kale S, Heinz MG. Noise-induced hearing loss increases the temporal precision of complex envelope coding by auditory-nerve fibers. Front Syst Neurosci 8: 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes LF, Turner JG, Parrish JL, Caspary DM. Processing of broadband stimuli across A1 layers in young and aged rats. Hear Res 264: 79–85, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Christopherson L. Speech identification difficulties of hearing-impaired elderly persons: the contributions of auditory processing deficits. J Speech Hear Res 34: 686–693, 1991. [DOI] [PubMed] [Google Scholar]
- Humes LE, Roberts L. Speech-recognition difficulties of the hearing-impaired elderly: the contributions of audibility. J Speech Hear Res 33: 726–735, 1990. [DOI] [PubMed] [Google Scholar]
- Juarez-Salinas DL, Engle JR, Navarro XO, Recanzone GH. Hierarchical and serial processing in the spatial auditory cortical pathway is degraded by natural aging. J Neurosci 30: 14795–14804, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killion MC, Niquette PA, Gudmundsen GI, Revit LJ, Banerjee S. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J Acoust Soc Am 116: 2395–2405, 2004. [DOI] [PubMed] [Google Scholar]
- Klatt DH. Software for a cascade/parallel formant synthesizer. J Acoust Soc Am 67: 971–995, 1980. [Google Scholar]
- Lash A, Rogers CS, Zoller A, Wingfield A. Expectation and entropy in spoken word recognition: effects of age and hearing acuity. Exp Aging Res 39: 235–253, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Middlebrooks JC. Auditory cortex spatial sensitivity sharpens during task performance. Nat Neurosci 14: 108–114, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JJ, Maxfield ND, Pitt GJ, Gonzalez VB. Auditory evoked response to gaps in noise: older adults. Int J Audiol 50: 211–225, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo SK, Grose JH, Buss E. Speech-evoked ABR: effects of age and simulated neural temporal jitter. Hear Res 333: 201–209, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699, 2005. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. National Institutes of Health Toolbox Cognition Battery (NIH Toolbox CB). Monogr Soc Res Child Dev 78: 1–172, 2013. [DOI] [PubMed] [Google Scholar]
- Overton JA, Recanzone GH. Effects of aging on the response of single neurons to amplitude modulated noise in primary auditory cortex of Rhesus macaque. J Neurophysiol 115: 2911–2923, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience offsets age-related delays in neural timing. Neurobiol Aging 33: 1483.e1481–1483.e1484, 2012. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience 192: 619–630, 2011. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Cunningham PA, Bartlett EL. Age-related differences in auditory processing as assessed by amplitude-modulation following responses in quiet and in noise. Front Aging Neurosci 2: 152, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci 31: 12638–12643, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults' comprehension of spoken sentences: age differences in resource allocation and connectivity. Cereb Cortex 20: 773–782, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA. Masking-level differences in the elderly: a comparison of antiphasic and time-delay dichotic conditions. J Speech Hear Res 34: 1410–1422, 1991. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am 97: 593–608, 1995. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Macdonald E, Pass HE, Brown S. Temporal jitter disrupts speech intelligibility: a simulation of auditory aging. Hear Res 223: 114–121, 2007. [DOI] [PubMed] [Google Scholar]
- Presacco A, Jenkins K, Lieberman R, Anderson S. Effects of aging on the encoding of dynamic and static components of speech. Ear Hear 36: e352–e363, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S. Evidence of degraded representations of speech in noise, in the aging midbrain and cortex. J Neurophysiol. (August 17, 2016). doi: 10.1152/jn.00372.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Jacoby LL, Sommers MS. Frequent false hearing by older adults: the role of age differences in metacognition. Psychol Aging 27: 33–45, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Wingfield A. Stimulus-independent semantic bias misdirects word recognition in older adults. J Acoust Soc Am 138: El26–E130, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Schneider B, Snyder JS, Alain C. Biological markers of auditory gap detection in young, middle-aged, and older adults. PLoS One 5: e10101, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Särelä J, Valpola H. Denoising source separation. J Mach Learn Res 6: 233–272, 2005. [Google Scholar]
- Schatteman TA, Hughes LF, Caspary DM. Aged-related loss of temporal processing: altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience 154: 329–337, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Hamstra SJ. Gap detection thresholds as a function of tonal duration for younger and older listeners. J Acoust Soc Am 106: 371–380, 1999. [DOI] [PubMed] [Google Scholar]
- Slee SJ, David SV. Rapid task-related plasticity of spectrotemporal receptive fields in the auditory midbrain. J Neurosci 35: 13090–13102, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Teismann IK, Manemann E, Lutkenhoner B. Auditory temporal processing in healthy aging: a magnetoencephalographic study. BMC Neurosci 10: 34, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Role of corticofugal feedback in hearing. J Compar Physiol A Neuroethol Sens Neural Behav Physiol 194: 169–183, 2008. [DOI] [PubMed] [Google Scholar]
- Surprenant AM. Effects of noise on identification and serial recall of nonsense syllables in older and younger adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14: 126–143, 2007. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhu X, Ding B, Walton J, Frisina R, Su J. Age-related hearing loss: GABA, nicotinic acetylcholine and NMDA receptor expression changes in spiral ganglion neurons of the mouse. Neuroscience 259: 184–193, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol 114: 1332–1343, 2003. [DOI] [PubMed] [Google Scholar]
- Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging 24: 761–766, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, O'Kane G, Wingfield A. Distraction by competing speech in young and older adult listeners. Psychol Aging 17: 453–467, 2002. [DOI] [PubMed] [Google Scholar]
- Vaden KI Jr, Kuchinsky SE, Ahlstrom JB, Dubno JR, Eckert MA. Cortical activity predicts which older adults recognize speech in noise and when. J Neurosci 35: 3929–3937, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese L, Bharadwaj HM, Shinn-Cunningham BG. Evidence against attentional state modulating scalp-recorded auditory brainstem steady-state responses. Brain Res 1626: 146–164, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O'Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci 18: 2764–2776, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience 160: 227–239, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CM, Rogers CS, Van Engen KJ, Peelle JE. Effects of age, acoustic challenge, and verbal working memory on recall of narrative speech. Exp Aging Res 42: 126–144, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC. Cognition assessment using the NIH Toolbox. Neurology 80: S54–S64, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Ettlinger M, Sheppard JP, Gunasekera GM, Dhar S. Neuroanatomical characteristics and speech perception in noise in older adults. Ear Hear 31: 471–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Garcia E. The Wechsler Abbreviated Scale of Intelligence (WASI). New York: Psychological Corp, 1999. [Google Scholar]