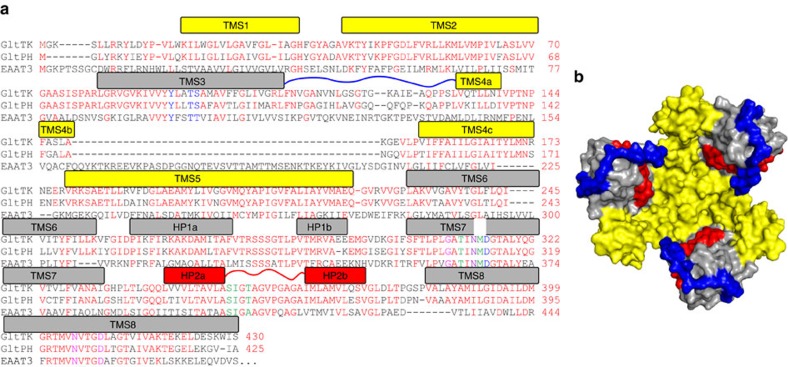
Figure 1. Overview of the structure of GltTk.
(a) Sequence alignment of the aspartate transporters GltTk form Thermococcus kodakarensis and GltPh from Pyrochoccocus horikoschii and the glutamate transporter EAAT3 from Rattus norvegicus. Identical residues between the archaeal proteins colored red, those involved in Na+1 binding in magenta, Na+2 in green and Na+3 in blue respectively. N313 involved in coordination of both Na+1 and Na+3 is in purple. Bars above the sequences indicate helical segments and are colored in yellow and grey for the trimerization and transport domain, respectively. Loop 3–4 is indicated by the blue line, HP2 in red. (b) Crystal structure of the substrate-loaded aspartate transporter GltTksub viewed from the extracellular side of the membrane, shown as surface. The trimerization domains in yellow, transport domains in gray, HP2 in red and long loop 3–4 in blue.