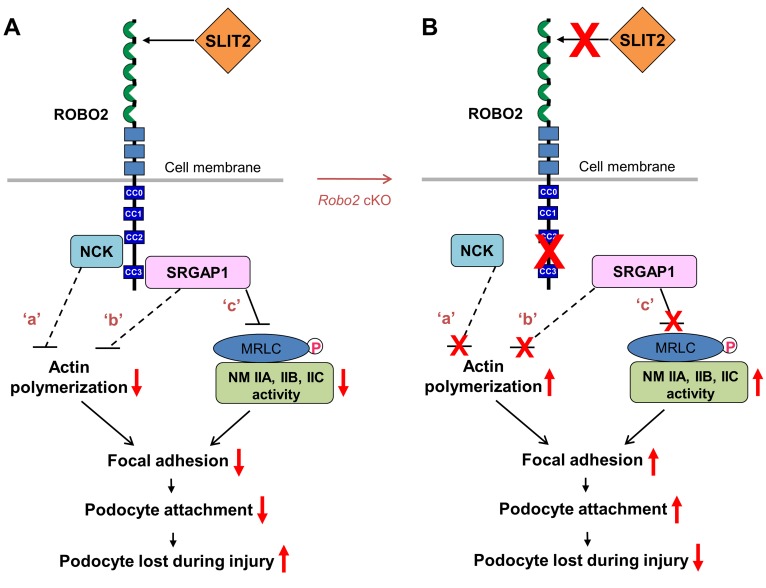
Figure 6. The proposed model: SLIT2/ROBO2 signaling destabilizes podocyte adhesion and attachment.
(A) ROBO2 is predominantly localized on the basal membrane of podocytes, and SLIT2 is secreted by podocytes (7) (also see Supplemental Figure 3). Upon SLIT2 binding, ROBO2 increases its binding to adaptor protein NCK (7) which, in turn, interacts with N-WASP and Arp2/3 to inhibit actin polymerization (62) (signaling ‘a’). ROBO2 also recruits SRGAP1, which inactivates the small GTPase Cdc42 to block actin polymerization (14) (signaling ‘b’). The SH3 domain of SRGAP1 binds to ROBO2, and its F-BAR domain is associated with nonmuscle myosin IIA (NMIIA)/IIB/IIC through myosin regulatory light chain (MRLC). The complex of ROBO2/SRGAPs/MRLC/NMII results in inactivation of NMII, possibly due to structurally blocking MRLC phosphorylation (signaling ‘c’). F-actin polymerization and NMIIA activity are required for normal focal adhesion formation and maturation (2, 26). Inactivation of actin polymerization and inhibition of NMII might interfere with focal adhesion assembly and result in a decrease in podocyte attachment. Signaling ‘a’: published data from Fan et al. (7); signaling ‘b’: published data from Wong et al. (14); signaling ‘c’: data from this study. Dashed lines indicate indirect inhibition while the solid line shows the direct inhibition. Red arrows indicate enhanced or inhibited activities. CC0–3, cytoplasmic conserved regions 0–3. (B) Removal of the SLIT2/ROBO2 inhibitory signaling in podocytes (e.g., in Robo2 cKO mice) promotes focal adhesion formation and podocyte attachment, which protects against podocyte loss during injury.